Første AIDS tilfellene beskrevet i den vestlige verden (familie med 3 personer/ døde i 1976)! Pasientene kom fra Tønsberg, Norge.
Tysk fjernsyn lagde for en del år siden program angående dette, og hadde også intervju med leder av barneavdelingen, Sykehuset i Vestfold, Karl Wilhelm Wefring. (Tror TV-kanalen var: sat1.de. Hvis noen finner opptaket er jeg takknemlig!).
https://admin.mekke.no/data/downloads/2537/_2004.pdf
Agder Vitenskapsakademi Årbok 2004. Se sidene 71 til 88, Christian Fredrik Lindboe, Sørlandet Sykehus HF
https://www.michaeljournal.no/i/2010/02/Da-hiv-kom-til-Norge
Det norske medicinske Selskab
Pathology of Fungal Infection Julintorn Somran, MD. .pdf
http://www.med.nu.ac.th/pathology/405313/book56/fungal-infection56.pdf
Classification of Fungi. American Society For Microbiology
A guide to the histological identification of
fungi in tissues
P. P. ANTHONY
From the Bland-Sutton Institute ofPathology, The Middlesex Hospital, London
Jeannette Guarner1
* and Mary E. Brandt2
Department of Pathology and Laboratory Medicine, Emory University, Atlanta, Georgia,1 and Mycotic Diseases Branch,
Centers for Disease Control and Prevention, Atlanta, Georgia2
https://link.springer.com/book/10.1007/978-3-030-06088-6
Fungal Infections of the Central Nervous System
Pathogens, Diagnosis, and Management
Abstract Springer Link
During the last 30 years, advances in intensive and critical care units, organ transplantation, concomitant use of immunosuppressive drugs, and increasing prevalence of chronic diseases, malnutrition, and other debilitating conditions, as well as the human immunodeficiency virus pandemic, have increased the incidence of systemic mycotic diseases, the most serious form of fungal diseases are the ones that comprise the central nervous system, representing the most dangerous clinical situations. In those cases, starting an adequate therapy through a rapid and assertive diagnosis is absolutely necessary. Considering the fastidious microbiological nature of some fungi (longtime requirement, specific culture conditions, and biohazard issues), as well as the lack of alternative testing availability, a rapid diagnosis is always challenging. When a tissue or liquid specimen is available, its pathological analysis constitutes a rapid and cost-effective way to provide a presumptive or definitive diagnosis of an invasive fungal infection; however, microbiologists, pathologists, and clinicians need to be aware of the limitations of microscopical diagnosis. In this chapter, we review the usual histological presentation of the most frequent central nervous system fungal infections.
Keywords
Aspergillus species Blastomyces dermatitis Central nervous system Candida species Coccidioides species Cryptococcus species Histopathology Histoplasma species Mucor species Neuropathology Rhizopus species
Abbreviations
- AIDS
-
Acquired immunodeficiency syndrome
- BAL
-
Broncoalveolar lavage
- CNS
-
Central nervous system
- CSF
-
Cerebrospinal fluid
- HIV
-
Human immunodeficiency virus
- PAS
-
Periodic acid-Schiff
6.1 Introduction
As a result of advancements in transplantation and the concomitant use of immunosuppressive drugs, the human immunodeficiency virus (HIV) pandemic, the high prevalence of chronic diseases such as diabetes, the incidence of invasive fungal infections, and central nervous system (CNS)-involvement have increased during the last decades (Raman-Sharma 2010; Brumble et al. 2017). Nevertheless, CNS compromise remains uncommon, but its associated morbidity and mortality are quite high. Even for immunocompetent hosts, the cure rate for patients receiving antifungal therapy for cryptococcal meningitis is around 75% and is only 25% for aspergillosis and mucormycosis (Perdigao et al. 2004).
Fungal infections have been recognized historically, but CNS fungal infections have been recognized recently. The first description of a fungal infection is attributed with Hippocrates, in his book Of the Epidemics (400 B.C.E.) (The Library of Victoria University, Toronto n.d.), in which he described white patches in the oral cavity of a debilitated patient (candidiasis). Zenkar in 1861 described a fatal case of intracerebral candidiasis, and Smith and Sano were the first reporting a case of Candida meningitis in 1933 (Segal and Elad 2010).
CNS fungal infections can be classified as parenchymal (i.e., abscess, granulomas, cerebritis), extra-axial (meningitis), or vascular (vasculitis), or in some cases a combination of them (Mathur et al. 2012). Fungi are ubiquitous in the environment, and CNS compromise often occurs after a pulmonary infection. Delay in diagnosis and management is a major complicating factor. Symptoms are nonspecific, and even patients with disseminated infection and multi-organ involvement may not develop organ-specific changes or clinical signs (Liu et al. 2011).
An Indian study including 130 histopathologically confirmed CNS fungal infection cases (Sundaram et al. 2006) reported that the most frequent pattern was granulomatous inflammation in the majority of patients (74 cases), followed by angioinvasion with infarcts and abscesses in 31 cases and angioinvasion with infarcts in 9 cases. Macroscopically, mycotic aneurysm with rupture and subarachnoid hemorrhage were seen in two patients, chronic meningitis in two, abscess in eight (single in three, multiple in five), and encephalitis with vasculitis and infarcts in four patients.
Fungal infections of the CNS could be caused by a large group of organisms; in addition, they are not including in a mandatory report disease by the public health authorities, and therefore, robust epidemiological information on their global burden is not available (Schwartz et al. 2018). However, according to reports from reference centers (Schwartz et al. 2018; Naggie and Perfect 2009), and the review in the present book, the most important and frequent entities include candidiasis, aspergillosis, cryptococcosis, mucormycosis, histoplasmosis, coccidioidomycosis, and blastomycosis.
Fungi are ubiquitous environmental organisms that may be unicellular (yeast), filamentous (molds), or show a dimorphic morphology. More than one million known mycotic species exist in nature, and around 200 species are known to be pathogenic for the human being. However, only about 20 fungal species produce invasive systemic infections, including CNS invasion (Guarner and Brandt 2011).
According to its morphology, fungi can be classified as follows: pseudomycetes/yeasts (Candida spp., Cryptococcus spp., Histoplasma spp., Blastomyces spp., Coccidioides spp., Paracoccidioides spp., and Sporotrichum spp.), septate mycetes (Aspergillus spp., Penicillium spp., Cephalosporium spp., Cladosporium spp., Diplorhinotrichum spp., Hormodendrum spp., and Paecilomyces spp.), and nonseptate mycetes (Mucor spp., Rhizopus spp., Absidia spp., Basidiobolus spp., Cunninghamella spp., and Mortierella spp.).
Dimorphic fungi such as Histoplasma spp., Coccidioides spp., Blastomyces spp., Sporotrichum spp., and Paracoccidioides spp. display a mycelial form at 25 °C (filamentous in nature) and transform into yeast (spherules) at normal human body temperature (37 °C). Encapsulated yeast, C. neoformans, preserves its morphology in normal human tissues and in the environment, similar to some septate and nonseptate mycetes.
In general, fungal infections are becoming more frequent because of expansion of at-risk populations (transplanted patients, those receiving immunosuppressive and chemotherapeutic agents, HIV-infected patients, premature infants, the elderly, and patients undergoing major surgery) (Table 6.1) and also because availability of treatment schemas permit longer survival of these patients (Naggie and Perfect 2009). Certain variations in the geographical distribution of endemic fungal infections can be attributed to climate changes, an extension of human habitats, ease of travel, and shifting populations. Therefore, during the last decades, a shift in the epidemiology of human mycoses has occurred (Guarner and Brandt 2011).
Table 6.1
Fungal pathogens of the CNS associated with specific conditions
|
Fungi |
|
|---|---|
|
Disease-induced immunosuppression |
|
|
HIV infection |
Cryptococcus neoformans |
|
Diabetes and iron overload |
Mucorales |
|
Hematological malignancies (e.g., acute leukemia) |
Aspergillus spp., and other molds |
|
Neutropenia (e.g., aplastic anemia) |
Aspergillus spp. |
|
Prematurity |
Candida spp. |
|
Drug-induced immunosuppression |
|
|
Corticosteroids |
Aspergillus spp., and other molds |
|
Biological drugs (TNFα inhibitors) |
Molds, dimorphic fungi, Cryptococcus spp. |
|
Immunomodulatory agents (e.g., ibrutinib) |
Aspergillus spp., Cryptococcus spp. |
|
Hematopoietic stem cell transplantation |
Aspergillus spp., non-Aspergillus molds |
|
Solid organ transplantation |
Candida spp., Aspergillus spp., non-Aspergillus molds |
|
Medical interventions |
|
|
Neurosurgery, spinal anesthesia, injection |
Aspergillus spp., other molds, Candida spp. |
|
Intravascular or intracranial devices |
Candida spp. |
|
Other |
|
|
Intravenous drug use |
Candida spp. |
Before the twenty-first century, bloodstream infections were mostly caused by Candida spp., and the most frequent invasive pulmonary infections included primarily aspergillosis and endemic mycoses. Nowadays, fungi previously not considered pathogenic, including mucoraceous genera (formerly called zygomycetes), and many hyaline and dematiaceous molds are frequently seen affecting immunocompromised patients. Therefore, diagnosis of infection versus colonization with these fungi is a common issue that has important treatment implications (Guarner and Brandt 2011). Moreover, advances in diagnostic imaging and specific patient support have permitted the possibility of collecting biopsy specimens for histological examination from sites previously not reachable; nevertheless, these advantages come with challenges related to the limited amount of tissue obtained and the architectural distortion produced by the procedure (Gavito-Higuera et al. 2016).
In histological terms, evidence of damaging tissue is a sufficient diagnostic proof of a CNS infection, even in patients with negative results in cultures and other tests. However, this demonstration requires a brain biopsy, which is one of the most important limitations (De Pauw et al. 2008). Frequently, fungi when present in tissue are not easily visible using routine stains; thus, Gomori methenamine silver or periodic acid-Schiff (PAS) staining can be used to improve their visibility and allow a better morphological identification (Schwartz et al. 2018; De Pauw et al. 2008). Some microscopic fungal characteristics can allow its identification to a genus level. For example, Aspergillus species can be differentiated from Mucorales by their septate, dichotomous branching hyphae, whereas Mucorales have an irregular shape of mostly uniseptate or pauciseptate hyphae. Some organisms show specific morphological characteristics, which can help in their identification. For example, H. capsulatum are small intracellular, budding yeasts; B. dermatitidis are thick-walled, broad-based budding yeasts; P. brasiliensis is big yeasts with small yeasts attached given a so-called pilot-wheel configuration; and Coccidioides species manifest themselves as large spherules that contain endospores (De Pauw et al. 2008; McCarthy et al. 2014).
Histopathological examination represents a rapid and cost-effective approach of providing a presumptive or definitive diagnosis of an invasive fungal infection. Nevertheless, microbiologists, pathologists, and clinicians need to be aware of the limitations of histological diagnosis, the pitfalls of morphological diagnosis, and the additional tests that can be performed (Guarner and Brandt 2011).
In this chapter, we review the most representative microscopic findings and interpretation pitfalls regarding the most frequently encountered fungi in CNS infections. We also present the complementary or alternative tests that can be performed in the biopsy specimen and other samples.
6.2 Disease Caused by Aspergillus spp.
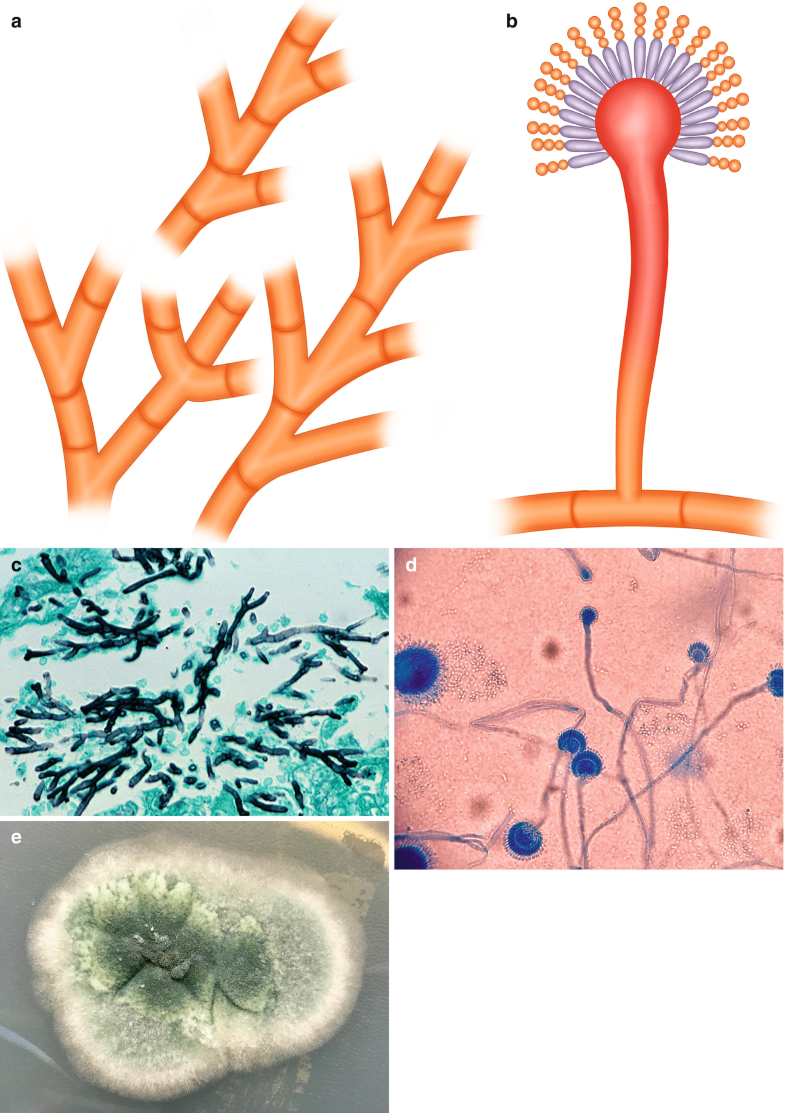
The genus Aspergillus is represented by molds (Fig. 6.1a, c (Courtesy of D. Palacios M.D.), e (Courtesy of Z. DL Rosa R.M.)) that reproduce asexually producing conidia (Fig. 6.1b, d (Courtesy of Z. DL Rosa R.M.)) (Bennett 2009). These fungi are ubiquitous and have been used for centuries for industrial proposes. This genus is able to survive in a variety of habitats from leaf litter to human tissues. The most frequent species associated with human disease is A. fumigatus, and other species, such as A. niger, cause disease in immunosuppressed individuals. Aspergillus fungi produce three particular clinical entities: allergic bronchopulmonary aspergillosis, chronic pulmonary aspergillosis/aspergilloma, and invasive or systemic aspergillosis (Riscili and Wood 2009).
Fig. 6.1
Aspergillus spp. (a) Illustration, septate acute-angle (45°)-branching hyphae. (b) Illustration, vesicle with conidia. (c) Septate acute-angle (45°)-branching hyphae, Gomory, 40×. (d) Vesicle with conidia from culture. (e) Appearance in culture. All illustrations are original by Jurado LF
Invasive pulmonary aspergillosis generally occurs in critically immunosuppressed patients, including patients with prolonged neutropenia, organ transplant recipients, patients with AIDS, premature newborns, and patients with chronic granulomatous disease (Sherif and Segal 2010). Apparition of neurological signs of stroke or seizures usually indicates that the fungus has reached the CNS.
Aspergillus spp. are one of the most frequent fungi isolated from neurological infections, most infections of the CNS are due to A. fumigatus, which gets into the CNS by hematogenous spread from the primary site of infection (lungs) or from contiguous anatomical sites, such as the paranasal sinuses (McCarthy et al. 2014). Focal lesions or a brain abscess are the predominant findings (Barrera-Herrera et al. 2015), while cerebral infarction caused by septic embolism, vascular thrombosis, or mycotic aneurysms are less frequent (McCarthy et al. 2014), and meningitis (without parenchymal involvement) is rarely reported (Antinori et al. 2013). Microscopically, Aspergillus spp. are thin (3–12 μm), septate, acute-angle (45°), or dichotomous branching hyphae (Fig. 6.1a, c), and when present in cavitary lesions, vesicles with conidia can be also identified (Fig. 6.1b, d).
In a retrospective study performed by Sundaram et al. (2006), 56% of the cases were caused by Aspergillus spp., 25% of them had a positive culture, and the majority of patients were immunocompetent. The most common source of spreading was continuity from sinuses, orbit or ear, followed by the hematogenous route; however, in eight cases, no source of infection was identified. Histologically, granulomas and dense fibrosis were the most frequent features. Eight cases presented isolated intracerebral granulomas; fibrosis was less marked in these lesions. The granulomas observed differed from tuberculous granulomas by the prominence of multinucleated giant cells, an abundance of neutrophils and plasma cells with lymphocytes and few epitheloid cells and marked fibrosis. Microscopic identification of the fungus was performed on GMS and PAS, and the culture positivity was 25%. The most common isolated organisms were A. flavus, A. fumigatus, A. niger, and A. terreus.
Lee et al. (2010) reported a series of 393 patients with evidence of fungal infection on histologic examination (a total of 231 (58.8%) were rhinocerebral infections); they analyzed the culture-histology concordance of filamentous fungi in 122 specimens; they showed concordance in 83% on cases with septated, acute-angle-branching hyphae and the presence of Aspergillus spp. in culture; Fusarium spp., Trichophyton spp., and others were recovered in culture from the discordant cases. Among the Aspergillus species isolated, A. fumigatus, A. flavus, A. niger, A. nidulans, and A. terreus were identified.
Cases of mixed infection, involving Aspergillus spp. and Candida or mucoraceous genera, have been described, posing an important dilemma. To be able to identify mixed infections, it is crucial to use alternative diagnostic testing (Hofman et al. 2010). In cases of invasive pulmonary aspergillosis, cultures are positive in only 50% of bronchoalveolar lavage (BAL) fluid specimens, and organisms recovered from BAL fluid samples could reflect colonization rather than the actual infection. In cases of invasive disease, isolation of Aspergillus spp. in blood cultures is approximately 5% (Sherif and Segal 2010).
Regarding the complementary test that can be performed when a case of aspergillosis is suspected, galactomannan and (1 → 3)-β-d-glucan, which are components of the fungi cell wall, can be measured in body liquids using commercially available kits (Sherif and Segal 2010). However, this analysis presents false-positive results in approximately 50% of individuals taking antibiotics (piperacillin, amoxicillin) and all patients receiving substances that contain products of A. niger fermentation (plasmalyte). This test also presents cross-reaction with other fungi, such as Penicillium spp. and Histoplasma spp. (Guarner and Brandt 2011). A study (Chong et al. 2016) that evaluated galactomannan testing in CSF from 17 patients with CNS Aspergillus infection and 27 controls reported a sensitivity of 88% and a specificity of 96%, which indicates a high diagnostic performance.
The (1 → 3)-β-d-glucan is present in a broad range of fungi (Alexander et al. 2010). There are commercially available assays for testing circulating (1 → 3)-β-d-glucan; it has been detected in patients with systemic fungal infections (invasive aspergillosis, candidemia, and Pneumocystis pneumonia) (Persat et al. 2008). The evidence on CNS infection is scarce; recently a study showed that its measurement in CSF represents a good approach for diagnosing and therapeutic monitoring of CNS fungal infection in children (Salvatore et al. 2016).
6.3 Disease Caused by Candida spp.
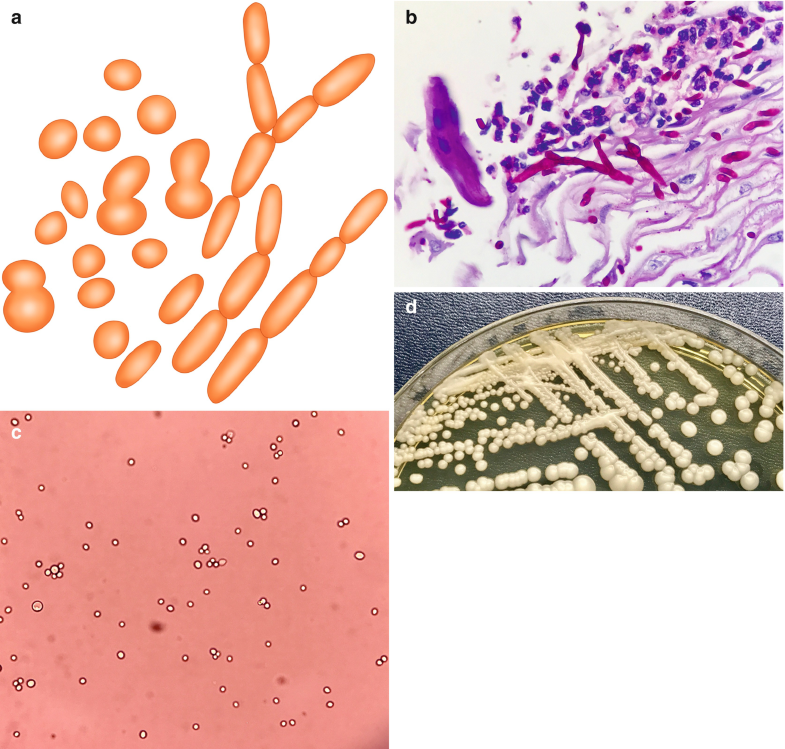
Candida spp. are small (4–6 μm), oval, thin-walled yeast-like fungi, which reproduce by budding or fission (Fig. 6.2a–c (Courtesy of Z. DL Rosa R.M.)). On culture media, Candida spp. form smooth, creamy white, glistening colonies (Fig. 6.2d (Courtesy of Z. DL Rosa R.M.)). The genus Candida is represented by over 2000 species, but only a few cause diseases in humans. Although more than 17 different Candida species have been reported as pathogens, the majority of invasive infections are attributed to 5 species: Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei (Vazquez and Sobel 2011).
Fig. 6.2
Candida spp. (a) Illustration, budding yeasts and thin branching, pseudohyphae or filaments showing periodic constrictions. (b) Pseudohyphae over tissular inflammatory reaction H&E, 100×. (c) Budding yeasts from culture. (d) Appearance in culture. All illustrations are original by Jurado LF
During the last decades, Candida species have evolved from infrequent to relevant and common human pathogens causing a wide spectrum of clinical syndromes. Candida albicans usually colonizes the oropharynx and vagina, and viable organisms can be cultured from these surfaces (Southern et al. 2008). When there are microbial imbalances caused by antibiotic use, hormonal deregulations, and immunosuppression (HIV infection, diabetes), superficial infections in the gastrointestinal or genitourinary tract can occur (Concia et al. 2009). Invasive candidiasis occurs frequently as a healthcare-associated infection, and patients at risk include those under broad-spectrum antibiotic treatment, immunosuppressant drugs, those with vascular access devices, cancer diagnosis, and neutropenia (Darouiche 2009).
CNS infections caused by Candida species usually arise from hematogenous spread, and most of these are caused by Candida albicans (Kullberg and Arendrup 2015). Epidemiological data on the Candida species distribution in CNS infections is not available; in the published series, Candida spp. usually appears in the second or third place of frequency (Brumble et al. 2017; Sundaram et al. 2006). Meningitis is the most common clinical form of CNS involvement due to Candida spp., but chronic meningitis, brain abscess formation, ventriculitis, mycotic aneurysms, and vasculitis have also been reported (Zimmermann et al. 2016; Merwick et al. 2015; Fennelly et al. 2013).
Candida organisms can form mats of budding yeasts and thin branching pseudohyphae, also called filaments, that may show periodic constrictions (Fig. 6.2a). The organisms can be seen with H&E (Fig. 6.2b), GMS, and PAS stains, but C. glabrata does not produce filaments. During histopathologic examination, it is very important to identify invasion of tissues and vessels, considering that isolation from the skin, lungs, and the gastrointestinal or genitourinary tract may be indicative of colonization (Southern et al. 2008; Darouiche 2009).
The usual tissue reaction, both in superficial and invasive disease, consists of neutrophilic inflammation with few lymphocytes and macrophages, fibrin, and coagulative necrosis (van de Veerdonk et al. 2010); sparse giant cells and granulomas can also be found. Due to the bloodstream nature of the infection, mycotic aneurysms or thrombophlebitis can develop. In candidemia cases, necrotizing vasculitis has been described; interestingly, organisms are not observed in affected vessels, supporting the idea that Candida soluble fractions are responsible for this pathogenic pattern (Sargent et al. 2010). In neutropenic patients, the necrosis is frequently accompanied by hemorrhage, and few lymphocytes and macrophages can be seen (Schuetz and Walsh 2015).
Candida spp. are yeasts that produce pseudohyphae (Fig. 6.2a, b), thus requiring differentiation from other yeasts and molds that produce hyphae in tissues, such as Aspergillus spp. and Trichosporon spp. Elongated Candida pseudohyphae can appear to be branching but can be differentiated because pseudohyphae are thin and do not have septations (Fig. 6.2b). Another pitfall is the germinating blastospores that appear to be branching but can be distinguished due to the absence of a constriction between the base of the blastospore and the germ tube (Guarner and Brandt 2011; Schuetz and Walsh 2015).
When the invasive disease is suspected, blood cultures are the most important tool for diagnosis; after all, blood culture positivity is around 50–70% of tested cases. Additionally, the peptide nucleic acid fluorescent in situ hybridization assay (PNA FISH) can be used to identify the most common Candida species in smears made from positive blood culture bottles (Posch et al. 2017).
Another diagnostic option for invasive infection is the multiplex-tandem PCR, which can be performed in whole blood, serum, or plasma and has yielded better and faster results compared with blood culturing; nevertheless, this methodology is still for research use only and needs to be validated for diagnostic purposes (Lau et al. 2010).
6.4 Disease Caused by Rhizopus spp. and Mucor spp.
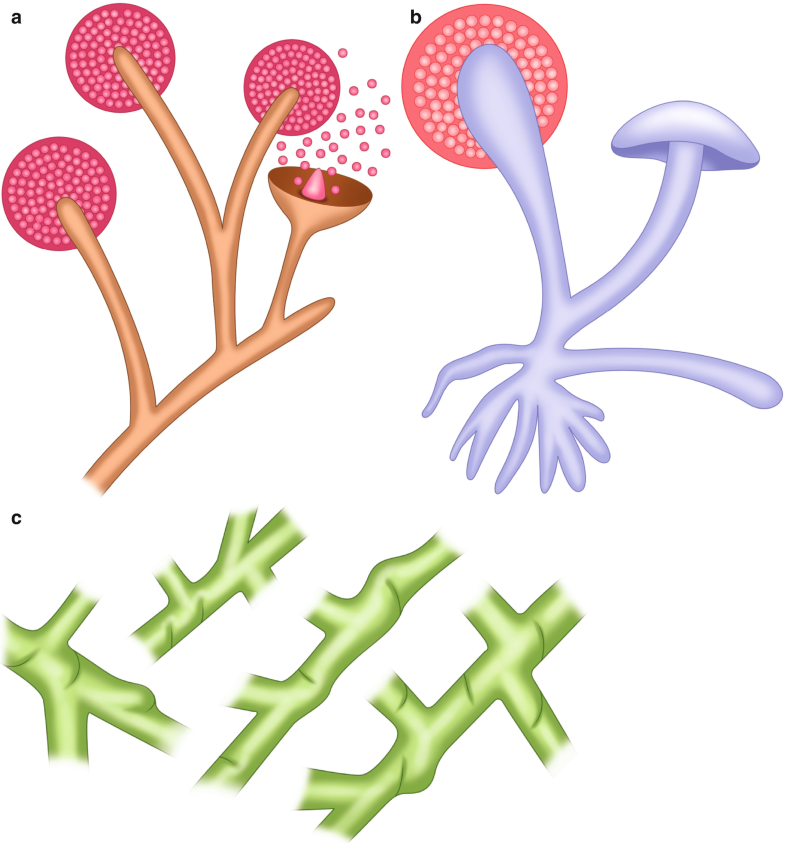
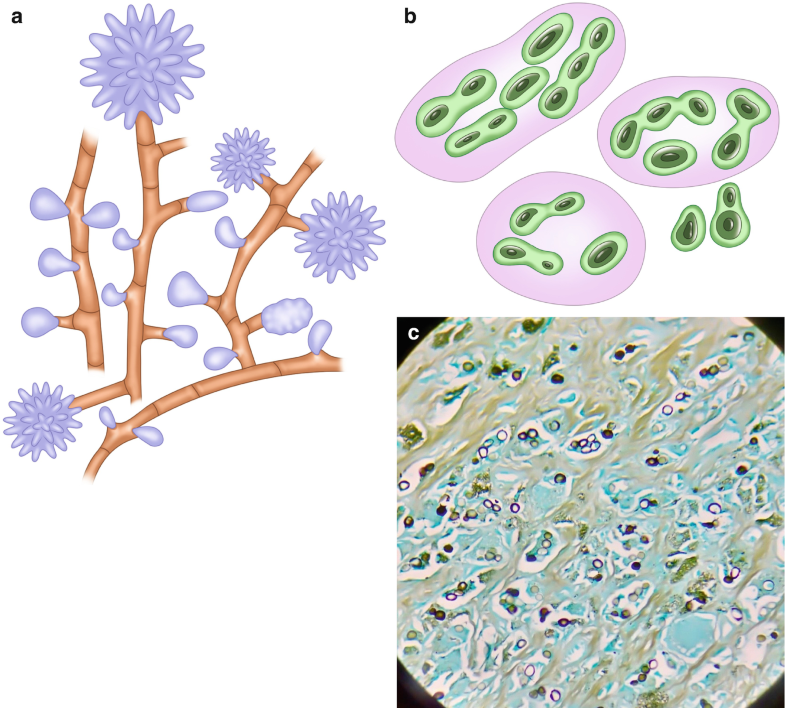
Since the 1800s, researchers have investigated a group of entities caused by ribbon-like, pauciseptate, hyaline molds (Fig. 6.3a–e (Courtesy of A. Agudelo M.D.)); however, the nomenclature of these fungi has not been completely defined (Ribes et al. 2000). Historically, these molds have been called Zygomycota (not used anymore) and Mucorales; therefore, the disease is known as mucormycosis or zygomycosis. Currently, it is accepted that the subphylum Mucoromycotina has two orders, the Mucorales and the Entomophthorales. The former was originally identified as affecting insects and causing mucocutaneous disease in immunocompetent human hosts, while the Mucorales cause a spectrum of predominantly angioinvasive disease in immunosuppressed patients (Fig. 6.3f (Courtesy of A. Agudelo M.D.)). Among the Mucorales, Rhizopus is the most frequent genus that causes human disease; conversely, Mucor spp. produce disease in less than 20% of cases (Naggie and Perfect 2009).
Fig. 6.3
Mucorales. (a) Illustration, usual appearance of Mucor spp. (b) Illustration, usual appearance of Rhizopus spp. (c) Illustration, hyphae with some septations (pauciseptate) and a 90° angle branching. (d) Rhizopus spp. from culture
Mucorales are ubiquitous in the environment and are usually found in soil and decomposing organic matter (Ribes et al. 2000). Their spores are easily airborne, frequently causing contamination in the laboratory; considering this, isolation of these molds must be correlated with the clinical history, in order to define their clinical significance. Inhaled spores can produce respiratory disease in immunosuppressed individuals, and the spores can also invade the skin and subcutaneous tissue by traumatic inoculation, contaminated needles, and even insect bites; less commonly, spores can be swallowed and cause gastrointestinal disease (Prabhu and Patel 2004).
When the immune response is not able to control the initial infection, the spores germinate and invade the surrounding tissue. Initially, there is an edematous reaction, and by the time the hyphae invade blood vessels, (Fig. 6.3f) the tissue becomes necrotic and acquires a characteristic black color. By contrast, immunocompetent individuals develop an intense inflammatory response and can present with a mass in the skin, respiratory sinuses, or the gastrointestinal tract (Roilides et al. 2014).
In general terms, mucormycosis presents itself in three main clinical ways, which are rhinocerebral (Fig. 6.3g (Courtesy of A. Rueda M.D.)), pulmonary, and cutaneous. Mortality due to disseminated disease is extremely high, and it is influenced by the predisposing factors and the clinical presentation (Roden et al. 2005). Early identification of the initial infection site is imperative to start a proper surgical and antifungal treatment. Additionally, for diagnosis, tissue specimens should be both cultured and histopathologically analyzed.
Identification of these molds in tissues is very important since it allows distinguishing the presence of the fungi as a pathogen from a culture contaminant and also is indispensable to define the presence of blood vessel affectation (Fig. 6.3f). Mucorales genera are characterized by nonpigmented, wide (5–20 μm), thin-walled, ribbon-like hyphae with some septations (pauciseptate) and a 90° angle branching (Ribes et al. 2000) (Fig. 6.3c, e). The hyphae may vary in width, appear folded or crinkled, and be sparse or fragmented. In lesions exposed to air and in culture media, thick-walled spherical structures can form at the ends of the hyphae (Fig. 6.3a, b, d). Routine H&E stains may show only the cell wall without structure inside (Fig. 6.3e, f); in cytologic specimens, the hyphae can be identified using Papanicolaou and calcofluor white stains; and GMS and PAS can also help highlight the fungal wall (Naggie and Perfect 2009).
In some situations, the hyphae may look degenerated, and many of the characteristics may not be appreciated in the specimen. In these cases, the pathologist must describe the degenerate hyphal elements observed in the specimen. This identifies the tissue where the fungus is found, ruling out the common possibility of culture contamination.
In immunosuppressed patients, the hyphal elements are usually found immersed in abundant necrosis, hemorrhage, and blood vessel thrombosis (Ben-Ami et al. 2009). Another important diagnostic feature is the identification of fungal elements invading the blood vessel wall or inside the lumen (Fig. 6.3f). Neutrophilic inflammation could also be identified surrounding the lesion.
It is important to be careful in differentiating Mucorales from other fungi that produce nonpigmented hyphae in tissue (Aspergillus spp.), other hyaline septate molds (Fusarium and Scedosporium), and Candida spp. (Ribes et al. 2000). An important morphological pitfall is the presence of many septations and acute-angle branching, which suggest Aspergillus spp. or another hyaline septate mold, while the identification of yeasts and pseudohyphae formation should suggest Candida spp. When using GMS, poorly stained hyphae are observed, which should suggest mucormycosis. Therefore, specifically identifying Mucorales in tissues or detecting dual infections by Mucorales genera and other fungi, immunohistochemistry, in situ hybridization, or PCR can be very useful (Hofman et al. 2010).
Like in most mycotic infections, culture is indispensable for organism-specific diagnosis. Furthermore, during sample-processing, is important to handle the specimens carefully, since aggressive grinding of the tissue may render the fragile fungal elements nonviable (Ribes et al. 2000). Mucorales genera are fast-growing fungi, but unfortunately, the yield of cultures is low. Also, although serologic tests have been attempted, they are not recommended (Guarner and Brandt 2011).
The retrospective Indian study performed by Sundaram et al. (2006) included a total of 130 CNS fungal infection cases during a 17-year period; in the study, 30% of the cases (39 patients) were mucormycosis, and of these patients, 37 developed the rhinocerebral form. In three patients, the disease was limited to the CNS, and their clinical presentation was stroke-like and diffuse encephalopathy. The most frequent histologic pattern was hemorrhagic infarction with angioinvasion and neutrophilic infiltrates. And the identified fungal elements were hyaline hyphae, pale and nonseptate with irregular or right-angle branching (Fig. 6.3c, e). Cultures were performed in 15 (38%) patients and were positive in 33% (5 cases), and Rhizopus oryzae was the most frequently isolated organism.
6.5 Disease Caused by Histoplasma spp.
Histoplasma capsulatum is a cosmopolitan fungus that can be found in old buildings, caves, and soil rich in bird and bat droppings; nevertheless, there are areas of high concentration where the disease is endemic; these areas include the Ohio and Mississippi River valleys in the United States, some countries in Central and South America, southern Europe, areas in Africa, and southeastern Asia (Wheat et al. 2016; Colombo et al. 2011). In most areas of the world, human histoplasmosis is due to H. capsulatum var. capsulatum; nevertheless, in western and central regions of sub-Saharan Africa, the African clade of Histoplasma capsulatum, formerly named H. capsulatum var. duboisii, can be found as the etiological agent of the disease. Frequently, histoplasmosis occurs in outbreaks related with old buildings renovation/demolition, or when tourists visit caves, but most of these cases are sporadic (Loulergue et al. 2007).
As with most fungi that cause systemic disease, the infection spreads by airborne route. In other words, the human being gets infected of histoplasmosis by inhaling the microconidia, and the immediate clinical response depends on the amount of fungus inhaled and the immune response; individuals may show no symptoms, may have acute or chronic pulmonary disease, or may have a disseminated presentation (Bueno-Fischer et al. 2009; Kauffman 2009). After the conidia are inhaled, they are phagocytized by alveolar macrophages and change to the yeast phase (Fig. 6.4b, c). The organisms are able to survive inside macrophages for weeks, and the migration of macrophages to the lymph nodes facilitates its dissemination. The disseminated form can occur both after initial infection and as reactivation of latent infection in immunocompromised individuals (HIV-AIDS, hematologic cancer patients, solid organ transplanted individuals, corticosteroids, and tumor necrosis factor antagonists users) (Hage et al. 2010).
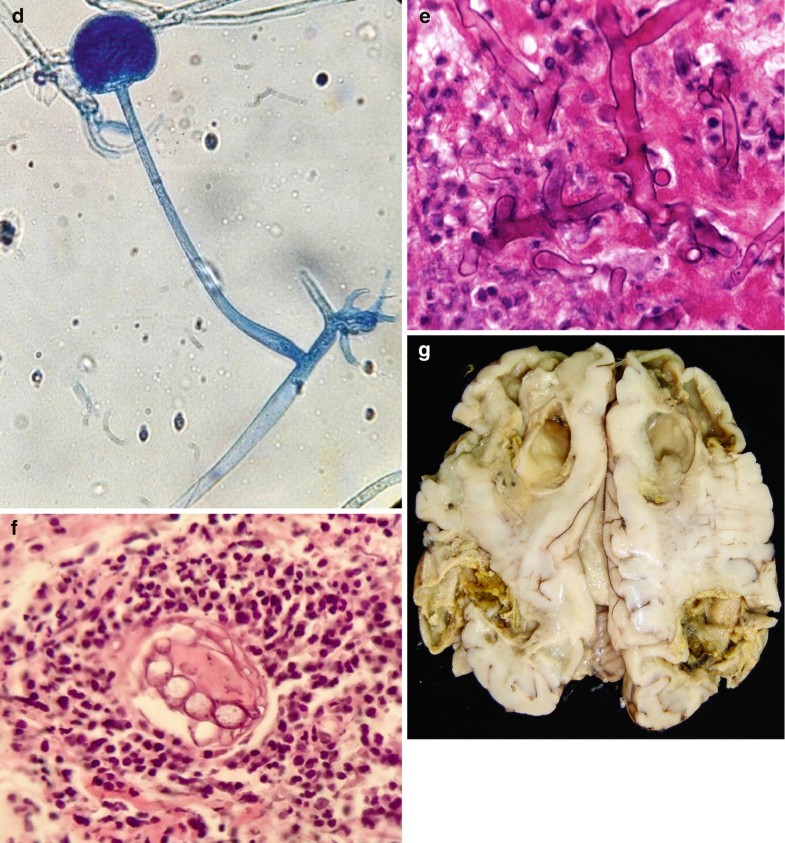
Fig. 6.4
Histoplasma spp. (a) Illustration, saprophytic form. (b) Illustration, parasitic form, yeast inside macrophages. (c) Multiple yeast infecting cells, Gomory, 40×. All illustrations are original by Jurado LF. (d) Nonpigmented, thin-walled, ribbon-like pauciseptate hyphae over necrotic tissue, H&E, 40×. (e) Fungal elements invading a blood vessel, H&E, 40×. (f) Macroscopic appearance of encephalic Mucormicosis, frontal lobes affected by hemorrhagic necrosis causing cavitary lesions. All illustrations are original by Jurado LF
Patients with high exposure loads or those who are immunosuppressed are at high risk of developing acute pneumonia or ARDS (Wheat et al. 2016; Bueno-Fischer et al. 2009). The migration of macrophages to mediastinal lymph nodes can make patients present with mediastinitis, and as macrophages travel through the body, the fungi spread to other organs, such as the skin, gastrointestinal tract, liver, spleen, and bone marrow; further, although infrequent, central nervous system compromise can occur (Wheat et al. 2016).
Histoplasma capsulatum var. capsulatum in tissue is an oval 2–4 μm yeast and may show narrow-based buds (Wheat et al. 2016) (Fig. 6.4b, c). With H&E staining, the basophilic yeast cytoplasm looks separated from the surrounding tissue by a clear zone corresponding to the cell wall, and using GMS and PAS stains, it is possible to highlight the cell wall. Due to the initial immune response, the yeasts are phagocytized by histiocytes, so they appear to be clustered; therefore, some authors suggest this as an important diagnostic clue (Fig. 6.4b, c). Further, this yeast aggregation inside macrophages and occasionally neutrophils is the usual presentation of Histoplasma in fluids stained with Papanicolaou or blood smears stained with Giemsa (Gupta et al. 2009).
There is scarce information regarding the histologic presentation of acute pulmonary histoplasmosis; some authors have described nodular areas of parenchymal and vascular necrosis associated with lymphohistiocytic vasculitis (Mukhopadhyay and Katzenstein 2010). This histopathologic pattern simulates the lymphomatoid granulomatosis but scattered small granulomas and the presence of yeasts must suggest the diagnosis of histoplasmosis.
The chronic pulmonary cases that radiographically appear as coin lesions show a granulomatous inflammation with the necrotic and calcified material, and it is common to find yeasts within the necrotic-calcified material (Wheat et al. 2016). When immunosuppressed patients develop disseminated disease, it is usual to observe sheets of macrophages filled with yeasts (Fig. 6.4b, c). The abundance of macrophages distorts the organ architecture and produces necrotic areas. Considering that the morphology of H. capsulatum is not specific, it is important to always perform clinical-epidemiologic correlation (Kauffman 2008).
Many fungi can be morphologically confused with H. capsulatum var. capsulatum when observed in tissue sections (Bueno-Fischer et al. 2009); for example, in the case of capsule-deficient cryptococci, size variation and weakly positive mucicarmine-stained yeasts may help to differentiate cryptococcosis from histoplasmosis. Another case could be the small variant of B. dermatitidis, where it is useful to look for the presence of broad-based budding and larger forms, which can help in making the diagnosis of B. dermatitidis infection. The endospores of Coccidioides spp. can also be a challenging case, where looking for rest of a ruptured spherule or an intact spherule is paramount for its differentiation. Another important example involves Pneumocystis jirovecii, as when distinguishing this organism, it is important to know that it lacks budding and has an intracystic focus. For the cases of Candida glabrata, this organism may show more size variability than histoplasmosis, and the inflammation is mostly neutrophilic. Finally, in Penicillium marneffei infection, it is important to keep in mind that this organism forms a transverse septum rather than a budding pattern.
In addition to the mentioned fungi, some parasites can also appear morphologically similar to Histoplasma spp., agents of leishmaniasis, toxoplasmosis, and Chagas’ disease, which also show intracellular organisms (Gupta et al. 2009). One of the most relevant histopathologic differences between these protozoans and Histoplasma is that H&E stains the entire organism, and none of them show the halo produced by the fungal cell wall. Kinetoplasts (a distinct hematoxylin-stained bar located to the side of the nucleus that represents a mass of mitochondrial DNA) should be observed in the cases of Leishmania or Trypanosoma infections. Finally, it is important to remember when toxoplasmosis and Chagas’ disease are suspected, that the infected cells are somatic (cardiomyocytes or neurons) rather than macrophages. In summary, to perform a definitive diagnosis of histoplasmosis from tissue sections is challenging, and if cultures were not requested, alternative testing must be demanded.
The culture of blood samples can help in diagnosing disseminated disease, but since Histoplasma spp. are an intracellular organism, lysis-centrifugation methods must be used to liberate the yeasts from infected cells. Additionally, this fungus has a long generation period, so cultures must be incubated for 4–6 weeks before being reported as negative (Wheat et al. 2016; Kauffman 2008). On the other hand, there are immune-based methods, where testing for antibodies can be performed using complement fixation or immunodiffusion; however, in immunodeficient patients, production of antibodies may not even occur (Kauffman 2009). In addition, false-positive serology results can occur in individuals with lymphoma, tuberculosis, and other fungal infections, especially in cases of blastomycosis (Wheat et al. 2016).
Through enzyme immunoassay, it is possible to detect certain antigens in urine and serum; after all, the antigen is concentrated in the urine, making Histoplasma antigen detection more reliable (Wheat et al. 2016). In the same way to antibody testing, there are false-positive results with antigen testing; particularly, the cross-reactivity with blastomycosis is problematic because histoplasmosis and blastomycosis have overlapping endemicity and histopathologically can have a striking resemblance. Furthermore, in patients with localized disease (nondisseminated), the antigen burden is lower, and thus sensitivity is lower. With those limitations, combining the results of detection of antigen in urine and serum may increase the sensitivity in patients with localized histoplasmosis (Swartzentruber et al. 2009).
6.6 Disease Caused by Coccidioides spp.
Coccidioidomycosis is a disease of the Western hemisphere caused by a dimorphic soil-dwelling fungus of the genus Coccidioides. It was first recognized as a clinical condition in Argentina in 1882, and, soon after, another case in the San Joaquin Valley in California (USA) was reported (Rixford and Gilchrist 1896).
There are two primary species that cause the disease. Coccidioides immitis is endemic in some parts of the United States, particularly in the California desertic areas (Adam et al. 2009); additionally, Coccidioides posadasii is present in desertic regions of the United States (Arizona, Utah, New Mexico, and West Texas), northwest Mexico, and desertic zones in Argentina, Paraguay, and areas of Central America (Colombo et al. 2011; Ampel 2009). Nevertheless, differences in morphology or clinical presentation have been found between the entities produced by each species. An interesting correlation between the incidence of the disease and specific environmental factors is commonly reported; for example, coccidioidomycosis incidence increases when there are rainy summers followed by dry winters, after earthquakes, or when humans establish themselves on the previously recognized endemic areas. Thus, when any of these situations take place, Coccidioides arthrospores are released in higher concentrations compared with the usual baseline (Parish and Blair 2008).
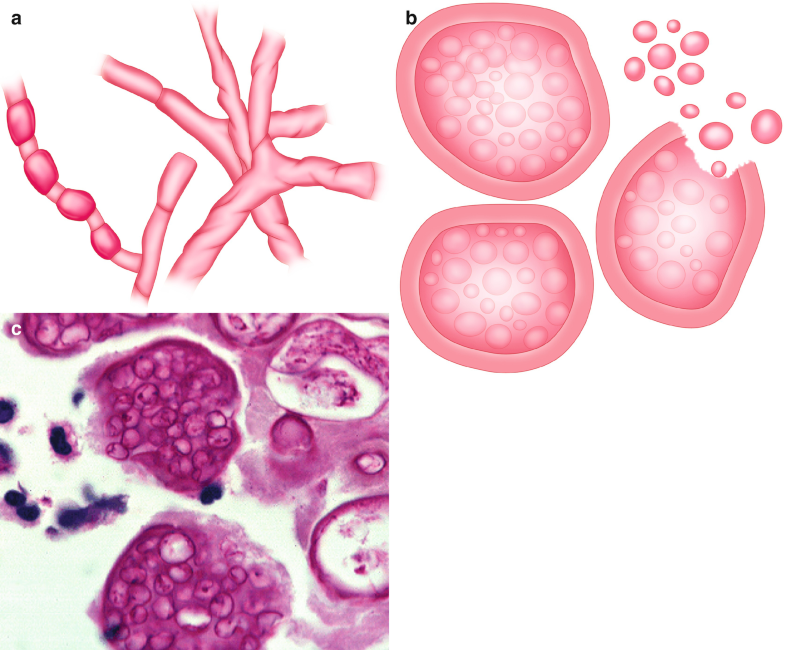
When susceptible individuals inhale the arthroconidia (Fig. 6.5a), this fungus reaches the alveoli and transforms into multinucleated spherical structures that contain hundreds of endospores (Fig. 6.5b, c (Courtesy of D. Palacios M.D.)) (Parish and Blair 2008). It is estimated that approximately 60% of infected people have no symptoms, while the remainder may present a clinical condition that simulates an acute community-acquired pneumonia, also known as “valley fever” (Adam et al. 2009; Ampel 2009). The chest X-rays may show lobar opacities and hilar adenopathy, and in some patients, cutaneous manifestations (erythematous rashes) are developed as reflections of the immune response to the acute infection (Gavito-Higuera et al. 2016).
Fig. 6.5
Coccidioides spp. (a) Illustration, saprophytic form, showing arthroconidia development. (b) Illustration, spherules with multiple endospores inside. (c) Spherules with multiple endospores inside. H&E, 40×. All illustrations are original by Jurado LF
Most of acute infections resolve with no complications; however, in a minority of patients, the infection may progress to a chronic condition, developing either a cavity or a nodule (Wheat et al. 2016). It is recognized that specific risk groups (African Americans, Asians, pregnant women, patients with diabetes, patients receiving corticosteroids) are more likely to develop disseminated disease (Adam et al. 2009). The most common sites of extrapulmonary involvement are the skin, lymph nodes, bones, and joints; nevertheless, the most feared is the extension to the CNS (Kauffman 2008). In those cases, the most frequent presentation is meningitis. Therefore, the usual imaging findings are meningeal enhancement and hydrocephalus, but focal brain lesions, infarcts, or areas of cerebritis or cerebellitis can also be observed (Lammering et al. 2013).
The most characteristic morphological feature of Coccidioides is the presence of spherules of diverse sizes (10–100 μm) with multiple endospores (2–5 μm); those can be identified with H&E staining (Fig. 6.5c) (Saubolle 2007). Sometimes, the walls of the spherules are ruptured, and the endospores can appear spilled over the surrounding tissue. It is usual that active lesions contain multiple organisms, while lesions in resolution show a lower number of fungal structures (Guarner and Brandt 2011).
Using GMS, it is possible to highlight spherule and endospore walls. In contrast, PAS stain affinity varies with age of the structures; therefore, immature endospores and spherules stain strongly, while mature structures appear less stained (Saubolle 2007). Occasionally, in the cavitary lung or cutaneous lesions, mycelia can be observed (Saubolle 2007). The sensitivity of histopathology for Coccidioides identification is 84% and 75% for cytology (Adam et al. 2009).
The predominant inflammatory response to endospores is neutrophilic, whereas the reaction to spherules is mostly granulomatous. Thus, early in the infection process, the histologic pattern tends to look mixed (pyogranulomatous) because the concentration of both fungal structures is high. In addition, lymphocytic clusters of B and T cells next to well-constituted granulomas with necrosis have been described and appear to be an important hallmark of coccidioidomycosis (Li et al. 2005). Eosinophil infiltrates can also be abundant, which produces eosinophilic major basic protein, creating the Splendore-Höeppli phenomenon (an intense cover of eosinophilic material around the fungal elements) (Read et al. 2005).
The microorganism to consider for differential diagnosis, is Rhinosporidium seeberi, a parasite that causes polyps in the upper respiratory tract, produces big sporangia (some can be seen with the naked eye) with multiple internal endospores. This parasite has very similar morphology to Coccidioides; however, its sporangia and endospores are bigger than the fungal spherules, and its internal sporangial wall stains with mucicarmine (Malo et al. 2014).
Considering that one important characteristic of Coccidioides is the presence of spherules, it is important to remember that endospores outside spherules or immature spherules without endospores can be confused with yeasts such as Blastomyces, Histoplasma, Candida, or Pneumocystis (Saubolle 2007). It also needs to be remembered that in immunosuppressed patients, more than one organism may coexist; thus, in areas of endemicity, Pneumocystis and Coccidioides could be found in the same specimen.
Detection of antibodies can be a helpful diagnostic tool. Nowadays, IgM and IgG are generally measured using EIA or immunodiffusion; however, it is also possible to use tube precipitation to measure IgM and complement fixation for IgG antibodies. False-negative results have been reported in up to 38% of patients with hematogenous infection and 46% of fatal cases (Adam et al. 2009). Detection of antigens in the urine using EIA has shown positive results in 71% of patients with coccidioidomycosis but has a cross-reaction in 10% of patients with other endemic mycoses (Durkin et al. 2008).
6.7 Disease Caused by Cryptococcus spp.
Human cryptococcosis is a systemic mycosis caused by some species of the Cryptococcus genus. Up to 40 species have been described, but few are recognized as a human pathogen (May et al. 2016). The most relevant are C. neoformans and C. gattii, but two other species, C. albidus and C. laurentii, have been reported in rare cases, producing disease in humans (Johnson et al. 1998; Kordossis et al. 1998).
These organisms are found in soil and are related with pigeon droppings. Infection involves most frequently the lungs and the CNS and less frequently can compromise the skin and the skeletal system. Because its incidence is high in immunocompromised patients, especially individuals with AIDS and organ transplant recipients, cryptococcosis is considered an opportunistic disease (May et al. 2016).
Cryptococcal meningoencephalitis is a frequent and life-threatening complication in patients with HIV infection (Williamson et al. 2017) and is the most common fungal infection of the CNS worldwide (223,100 estimated cases in 2014) (Rajasingham et al. 2017) causing a substantial disease burden in countries with poor access to medical care and high numbers of people living with HIV.
C. neoformans is the causative agent for the majority of infections in immunocompromised individuals, while C. gattii causes disease in immunocompetent hosts (Huston and Mody 2009). Additionally, C. neoformans var. grubii (serotype A) and C. neoformans var. neoformans (serotype D) have a worldwide distribution and are primarily associated with pigeon guano. HIV infection is the most important risk factor for cryptococcal disease; however, other conditions associated with cryptococcosis include chronic lung, liver, and renal disease, autoimmune diseases, immunosuppressant use, and cancer (Galanis and MacDougall 2010).
Irrespective of the species, inhaling cryptococcal yeasts or basidiospores infects humans; therefore, the lung is always the first infected organ (Huston and Mody 2009). In contrast to other pathogenic fungi, few exposed individuals remain asymptomatic; most develop pneumonia, cryptococcomas, or pleural effusion. From the lungs, cryptococci can disseminate to the CNS, resulting in meningitis or cryptococcomas. Further, C. gattii more frequently causes solid lesions in the lungs and brain than C. neoformans. The occurrence of the disseminated disease depends on the host immune status; in immunocompetent patients the most frequent presentation is pulmonary, while immunosuppressed patients commonly develop CNS disease (Williamson et al. 2017).
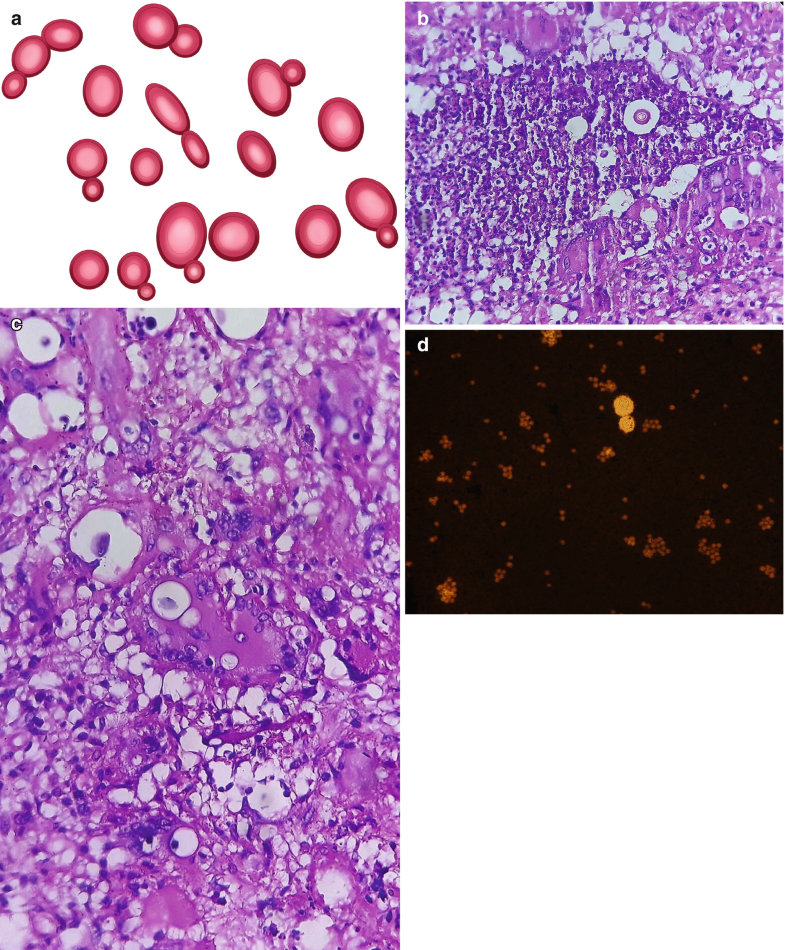
Cryptococcus spp. is a spherical to oval encapsulated yeast, measuring approximately 5–10 μm in diameter in clinical specimens with a narrow-based budding and a capsule ranging in size from 1 to >30 μm (Fig. 6.6a, d). In specimens isolated from the outside environment, yeast tends to be smaller and with a thinner capsule (Neilson et al. 1997). The thick, polysaccharide capsule gives the yeasts a characteristic appearance of having a clear space around them (Fig. 6.6b, c (Courtesy of M. Tuñon M.D.), d).
Fig. 6.6
Cryptococcus spp. (a) Illustration, narrow-based budding yeast. (b, c) Granulomatous reaction, multiple yeasts inside multinucleated giant cells, abundant lymphocytic infiltrate, H&E, 40×. (d) Yeast in CSF smear. Its thick polysaccharide capsule gives the yeasts a characteristic appearance of having a clear space around them. India ink, 40×. All illustrations are original by Jurado LF
When analyzing CSF, India ink is the ideal negative stain to highlight the capsule (Fig. 6.6d). Due to the capsule, the buds appear separate from the mother cell. The capsule can be stained with Alcian blue and Mayer’s or Southgate’s mucicarmine. As it happens with all other yeasts, the wall stains with GMS and PAS; moreover, for cryptococci, Fontana-Masson also results in a stain due to the presence of melanin (Williamson et al. 2017).
The histopathological reaction varies from well-formed granulomas, where the yeasts are found inside histiocytes and giant cells, to minimal inflammatory response with abundant extracellular organisms that distorts the tissue architecture (Gazzoni et al. 2009). When the granulomatous pattern is present, the granulomas show a spectrum from abundant necrosis to fibrosis (Fig. 6.6b, c). In some cases, the fibrosis is intense, with abundant stocky-spindle fibroblast cells in a storiform pattern accompanied by a background of lymphocytes and plasma cells, giving the appearance of an inflammatory pseudotumor (Sing and Ramdial 2007). Some authors have suggested that the histological reaction is related to the immune status of the patient and the presence or absence of capsule (Rajasingham et al. 2017).
In some cases, the yeast produces a thinner polysaccharide capsule; thus, these organisms may look similar to other yeasts, such as Candida spp. or Histoplasma. In this situation, staining these specimens with Fontana-Masson may show evidence of melanin, which is a hallmark of cryptococci. The use of cryptococcal antigen analysis in serum and CSF may not be useful in patients with poorly encapsulated cryptococci because most of the serologic tests detect capsular antigens (Gazzoni et al. 2009).
Cryptococcal antigen testing using latex agglutination or EIA can be performed in serum and CSF, showing a sensitivity and specificity of above 90%; nevertheless, false-negative results can occur due to a low fungal burden or a prozone phenomenon. Alternatively, false-positive results have been reported in infected individuals with Trichosporon spp. or Klebsiella pneumoniae, in those with a positive rheumatoid factor, or when the reagent was incubated with the specimen beyond the recommended time (Williamson et al. 2017). Cultures, using canavanine-glycine-bromothymol blue medium, that turn blue in the presence of C. gattii are helpful to identify the infective species and are indispensable for measuring antifungal susceptibility when indicated.
6.8 Disease Caused by Blastomyces dermatitidis
Gilchrist described blastomycosis in Baltimore (USA) during the 1890s as a skin infection caused by what he thought was a protozoan organism (Gilchrist 1894); thus, the disease was named as Gilchrist’s disease. Initially, because skin manifestations of blastomycosis were very striking, it was considered a dermatologic condition (Gilchrist and Stokes 1898). The concept of airborne transmission was not recognized until pathologic descriptions allowed the pathophysiologic mechanisms to be elucidated (Sarosi and Davies 1979). There are infrequent cases of direct cutaneous inoculation in laboratory workers and veterinarians, but virtually all cases of blastomycosis are considered to originate primarily from lung disease (Sarosi and Davies 1979).
The etiological agent is the dimorphic fungus Blastomyces dermatitidis. The organism exists in nature in the mold or mycelial phase and converts to the yeast phase in tissues at body temperature (Fig. 6.7a, b). The mold is the infective form, producing conidia that can be aerosolized and subsequently inhaled. In culture, B. dermatitidis grows from 25 to 28 °C as a mold and at 37 °C as a yeast (Nemecek et al. 2006).
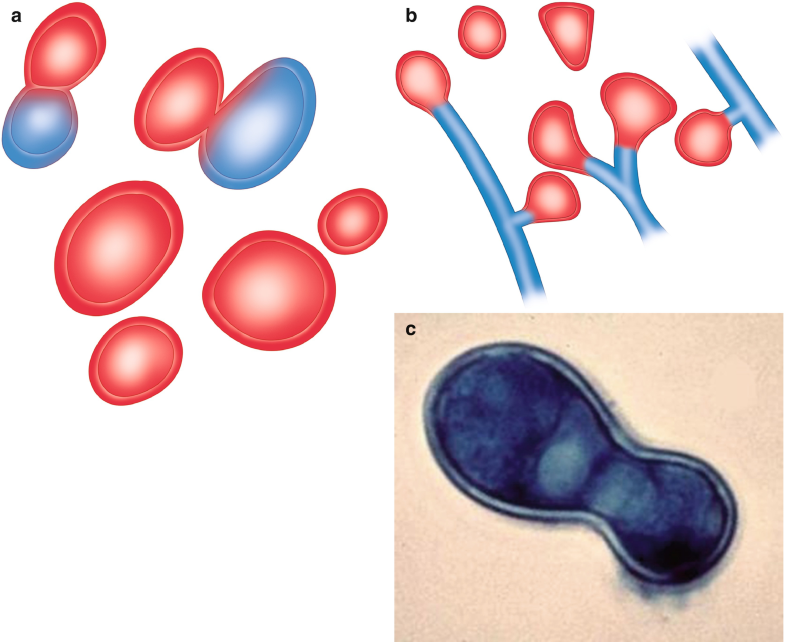
Fig. 6.7
Blastomyces dermatitidis. (a) Yeast form, with broad-based budding. (b) Mycelial or saprophytic form, forming single conidium showing its characteristic “lollipop” morphology. (c) Thick-walled yeast with broad-based budding, showing its “footprint” morphology. All illustrations are original by Jurado LF
This fungus has been isolated from soil in the Mississippi and Ohio River valleys, around the Great Lakes and the Saint Lawrence River, which include multiple states in the United States (southeastern, south central, and upper Midwestern states); it has also been found in several Canadian provinces (McKinnell and Pappas 2009). Outbreaks of blastomycosis after a source exposure have been reported, but most cases occur sporadically in the endemic areas. Occasionally, cases are reported in areas where the disease is not endemic, such as Colorado, Texas, Kansas, and Nebraska in the United States, and from other countries around the world (Shukla et al. 2009).
Patients infected with B. dermatitidis can develop pneumonia, extrapulmonary disease, or both. Lung involvement often imitates an acute bacterial pneumonia, lung cancer, or tuberculosis. Cutaneous lesions, which can be present as verrucous or ulcerative lesions, are the most common extrapulmonary manifestation, followed by bone, prostate, and CNS disease.
In the yeast phase, the organism appears as spherical, budding, thick-walled yeast with a daughter cell forming a single bud with a broad base. Further, size varies from 5 to 15 μm, and most are spherical to oval and have a double cell wall appearance, which consists of the interior and exterior components of its thick cell surface (Fig. 6.7c). The yeast may be found inside or outside of macrophages in the pyogranulomatous tissue response (McKinnell and Pappas 2009).
After inhalation of the conidia, a variety of responses can occur in the lungs, from asymptomatic disease to acute and chronic pneumonia, and fatal acute respiratory distress syndrome (McKinnell and Pappas 2009; Patel et al. 2010). In up to 40% of cases, the disease has become systemic at the time of diagnosis, affecting the skin, soft tissue, bone, genitourinary tract, or the CNS (McKinnell and Pappas 2009). Blastomycosis in immunocompromised hosts appears to be more severe and frequently fatal. In patients with CNS involvement, diabetes mellitus is an important risk factor (Bariola et al. 2010).
B. dermatitidis in tissue appears as yeasts that measure 8–15 μm in diameter, have a thick refractile cell wall, and usually show a single, broad-based bud (Fig. 6.7a, c). The yeasts can be identified in many samples, such as sputum, bronchoalveolar lavage, fine-needle aspirates, CSF, and biopsy specimens. When the H&E stain is used, the thick refractile cell wall gives the appearance of a space between the fungal cell contents and the surrounding tissue. The multiple nuclei of the yeast stain with hematoxylin. Sometimes, this fungus can show small yeast forms, called microforms. Additionally, B. dermatitidis can be identified with routinely used preparations such as Papanicolaou and KOH. The contour of the yeast is best appreciated with silver stains such as PAS or GMS (Patel et al. 2010; Lemos et al. 2000).
The histological reaction produced by the yeasts is mainly granulomatous with diverse degrees of neutrophilic infiltrate; therefore, as in the cases of coccidioidomycosis, it has been described as pyogranulomatous inflammation (Patel et al. 2010).
Few studies have compared the identification of broad-based budding yeasts in histopathologic or cytologic specimens with culture or other diagnostic methods that confirm the diagnosis of blastomycosis. Patel et al. (2010), in a retrospective study of 53 cases, reported that Coccidioides immitis, Candida albicans, and Aspergillus were recovered from 10% of the pathologic specimens, demonstrating broad-based budding yeasts in the direct histopathologic examination. A previous study by Lemos et al. (2000) of patients with blastomycosis showed that in a high percentage of the cultures, Candida was isolated. These results suggest that not all broad-based budding yeasts in the 8–15 μm diameter range are Blastomyces.
Considering that histopathologic and cytologic studies can provide results before the culture, there is pressure to use these findings to guide treatment, particularly because B. dermatitidis can take up to 3 weeks to grow or may not grow. The sensitivity of culture varies depending on the specimen that was obtained and may range from 62% to 100% (Lemos et al. 2000; Martynowicz and Prakash 2002). The diagnostic yield of histopathology will depend on the expertise of the pathologist (McKinnell and Pappas 2009). Because of the possibility of false-positive results, pathologists must describe the yeast and budding pattern observed in the tissue and also should add a commentary in the report about the yeasts that can have similar morphology. In addition, alternative tests should be performed to determine if the patient truly has blastomycosis, especially in cases from areas where the disease is not endemic or when the clinical presentation is not usual.
B. dermatitidis antigens can be detected in the urine and serum by using an EIA. The sensitivity and specificity have been reported to be above 90%; nevertheless, cross-reactivity occurs in patients with histoplasmosis, paracoccidioidomycosis, and penicilliosis caused by P. marneffei (Durkin et al. 2004). Because of the cross-reactivity, it is useful to perform antigen tests for both blastomycosis and histoplasmosis. Detection of antibodies to B. dermatitidis in serum using traditional complement fixation and immunodifusion has poor specificity and sensitivity; however, as antigens have been better purified and used in radioimmunodiffusion and EIAs, the sensitivity and specificity of serology are significantly higher (McKinnell and Pappas 2009).
6.9 Conclusion
Due to the high morbidity and mortality rates associated with systemic fungal infections and particularly in cases of CNS involvement, a rapid and accurate diagnosis is always mandatory. The broad spectrum in clinical and pathogenic presentation makes its diagnosis a big challenge. When a tissue or liquid specimen is available, the pathological analysis is a very useful tool for rapid and accurate diagnosis. Usually, pathological studies can provide results before the culture or other analyses are available. Nevertheless, it is important to remember the limitations of this approach because many of the fungal structures among different species are very similar. Therefore, it could be really easy to misdiagnose a specific fungal infection, which would have a relevant impact on therapy. Thus, it is always important to consider the clinical information, as well as complementary analysis, in order to obtain a definitive ethological diagnosis.
References
-
Adam RD, Elliott SP, Taljanovicc MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med. 2009;122:770–7.PubMedPubMedCentralGoogle Scholar
-
Alexander B, Smith P, Davis R, Perfect J, Reller L. The (1, 3)-D glucan test as an aid to the early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol. 2010;48(11):4083–8. https://doi.org/10.1128/JCM.01183-10.CrossRefPubMedPubMedCentralGoogle Scholar
-
Ampel NM. Coccidioidomycosis: a review of recent advances. Clin Chest Med. 2009;30:241–51.CrossRefGoogle Scholar
-
Antinori S, Corbellino M, Meroni L, Resta F, Sollima S, Tonolini M, Tortorano AM, Milazzo L, Bello L, Furfaro E, Galli M, Viscoli C. Aspergillus meningitis: a rare clinical manifestation of central nervous system aspergillosis. Case report and review of 92 cases. J Infect. 2013;66:218–38.CrossRefGoogle Scholar
-
Bariola J, Perry P, Pappas PG, Proia L, Shealey W, Wright PW, Sizemore JM, Matthew Robinson M, Bradsher RW. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50:797–804.CrossRefGoogle Scholar
-
Barrera-Herrera LE, Vera A, Álvarez J, Lopez R. Necrotizing encephalitis caused by disseminated Aspergillus infection after orthotopic liver transplantation. Case Rep Gastroenterol. 2015;9(1):1–6. https://doi.org/10.1159/000371541.CrossRefPubMedPubMedCentralGoogle Scholar
-
Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect. 2009;59:134–8.CrossRefGoogle Scholar
-
Bennett JW. Aspergillus: a primer for the novice. Med Mycol. 2009;47:S5–S12.CrossRefGoogle Scholar
-
Brumble LM, Reza MB, Dhakal LP, Cruz G, Saleh OMA. Fungal infections of the central nervous system: clinical, radiographic and laboratory manifestations. J Microbiol Exp. 2017;5(6):00167. https://doi.org/10.15406/jmen.2017.05.00167.CrossRefGoogle Scholar
-
Bueno-Fischer G, Mocelin H, Severo CB, Oliveira F d M, Xavier MO, Severo LC. Histoplasmosis in children. Paediatr Respir Rev. 2009;10:172–7.CrossRefGoogle Scholar
-
Chong GM, Maertens JA, Lagrou K, Driessen GJ, Cornelissen JJ, Rijnders BJ. Diagnostic performance of galactomannan antigen testing in cerebrospinal fluid. J Clin Microbiol. 2016;54:428–31.CrossRefGoogle Scholar
-
Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol. 2011;49:785–98.PubMedPubMedCentralGoogle Scholar
-
Concia E, Azzini AM, Conti M. Epidemiology, incidence and risk factors for invasive candidiasis in high-risk patients. Drugs. 2009;69(Suppl 1):5–14.CrossRefGoogle Scholar
-
Darouiche RO. Candida in the ICU. Clin Chest Med. 2009;30:287–93.CrossRefGoogle Scholar
-
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Mu-oz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. https://doi.org/10.1086/588660.CrossRefPubMedPubMedCentralGoogle Scholar
-
van de Veerdonk FL, Kullberg BJ, Mihai N. Pathogenesis of invasive candidiasis. Curr Opin Crit Care. 2010;16(5):453–45. https://doi.org/10.1097/MCC.0b013e32833e046e.CrossRefPubMedGoogle Scholar
-
Durkin MJ, Witt A, LeMonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol. 2004;42:4873–5.CrossRefGoogle Scholar
-
Durkin M, Connolly P, Kuberski T, Myers R, Kubak BM, Bruckner D, Pegues D, Wheat LJ. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis. 2008;47:e69–73.CrossRefGoogle Scholar
-
Fennelly AM, Slenker AK, Murphy LC, Moussouttas M, DeSimone JA. Candida cerebral abscesses: a case report and review of the literature. Med Mycol. 2013;51:779–84.CrossRefGoogle Scholar
-
Galanis E, MacDougall L. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis. 2010;16:251–7.CrossRefGoogle Scholar
-
Gavito-Higuera J, Mullins CB, Ramos-Duran L, Olivas Chacon CI, Hakim N, Palacios E. Fungal infections of the central nervous system: a pictorial review. J Clin Imaging Sci. 2016;6:24. https://doi.org/10.4103/2156-7514.184244.CrossRefPubMedPubMedCentralGoogle Scholar
-
Gazzoni AF, Severo CB, Salles EF, Severo LC. Histopathology, serology and cultures in the diagnosis of cryptococcosis. Rev Inst Med Trop Sao Paulo. 2009;51:255–9.CrossRefGoogle Scholar
-
Gilchrist TC. Protozoan dermatitis. J Cutan Genitourin Dis. 1894;12:496–9.Google Scholar
-
Gilchrist TC, Stokes WR. Case of pseudo-lupus vulgaris caused by Blastomyces. J Exp Med. 1898;3:53–78.CrossRefGoogle Scholar
-
Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–80. https://doi.org/10.1128/CMR.00053-10.CrossRefPubMedPubMedCentralGoogle Scholar
-
Gupta N, Arora S, Rajwanshi A, Nijhawan R, Srinivasan R. Histoplasmosis: cytodiagnosis and review of literature with special emphasis on differential diagnosis on cytomorphology. Cytopathology. 2009; https://doi.org/10.1111/j.1365–2303.2009.00693.x.
-
Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Wheat LJ. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker herapy. Clin Infect Dis. 2010;50:85–92.CrossRefGoogle Scholar
-
Hofman V, Dhouibi A, Butori C, Padovani B, Gari-Toussaint M, Garcia-Hermoso D, Baumann M, Vénissac N, Cathomas G, Hofman P. Usefulness of molecular biology performed with formaldehyde-fixed paraffin embedded tissue for the diagnosis of combined pulmonary invasive mucormycosis and aspergillosis in an immunocompromised patient. Diagn Pathol. 2010;5:1–7.CrossRefGoogle Scholar
-
Huston SM, Mody CH. Cryptococcosis: an emerging respiratory mycosis. Clin Chest Med. 2009;30:253–64.CrossRefGoogle Scholar
-
Johnson LB, Bradley SF, Kauffman CA. Fungaemia due to Cryptococcus laurentii and a review of non-neoformans cryptococcaemia. Mycoses. 1998;41:277–80.CrossRefGoogle Scholar
-
Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis. 2008;21:421–5.CrossRefGoogle Scholar
-
Kauffman CA. Histoplasmosis. Clin Chest Med. 2009;30:217–25.CrossRefGoogle Scholar
-
Kordossis T, Avlami A, Velegraki A, Stefanou I, Georgakopoulos G, Papalambrou C, Legakis NJ. First report of Cryptococcus laurentii meningitis and a fatal case of Cryptococcus albidus cryptococcaemia in AIDS patients. Med Mycol. 1998;36:335–9.CrossRefGoogle Scholar
-
Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373:1445–56.CrossRefGoogle Scholar
-
Lammering JC, Iv M, Gupta N, Pandit R, Patel MR. Imaging spectrum of CNS coccidioidomycosis: prevalence and significance of concurrent brain and spinal disease. AJR Am J Roentgenol. 2013;200:1334–46.CrossRefGoogle Scholar
-
Lau A, Halliday C, Chen SC, Playford EG, Stanley K, Sorrell TC. Comparison of whole blood, serum, and plasma for early detection of candidemia by multiplex-tandem PCR. J Clin Microbiol. 2010;48:811–6.CrossRefGoogle Scholar
-
Lee S, Yun NR, Kim KH, Jeon JH, Kim EC, Chung DH, Park WB, Oh MD. Discrepancy between histology and culture in filamentous fungal infections. Med Mycol. 2010;48:886–8.CrossRefGoogle Scholar
-
Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol. 2000;4:391–406.CrossRefGoogle Scholar
-
Li L, Dial SM, Schmelz M, Rennels MA, Ampel NM. Cellular immune suppressor activity resides in lymphocyte cell clusters adjacent to granulomata in human coccidioidomycosis. Infect Immun. 2005;73:3923–8.CrossRefGoogle Scholar
-
Liu X, Ling Z, Li L, Ruan B. Invasive fungal infections in liver transplantation. Int J Infect Dis. 2011;15(5):e298–304.CrossRefGoogle Scholar
-
Loulergue P, Bastides F, Baudouin V, Chandenier J, Mariani-Kurkdjian P, Dupont B, Viard JP, Dromer F, Lortholary O. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis. 2007;13:1647–52.CrossRefGoogle Scholar
-
Malo J, Luraschi-Monjagatta C, Wolk DM, Thompson R, Hage CA, Knox KS. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11(2):243–53. https://doi.org/10.1513/AnnalsATS.201308-286FR.CrossRefPubMedGoogle Scholar
-
Martynowicz M, Prakash UBS. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest. 2002;121:768–73.CrossRefGoogle Scholar
-
Mathur M, Johnson CE, Sze G. Fungal infections of the central nervous system. Neuroimaging Clin N Am. 2012;22(4):609–32.CrossRefGoogle Scholar
-
May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14(2):106–17. https://doi.org/10.1038/nrmicro.2015.6.CrossRefPubMedGoogle Scholar
-
McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med. 2014;371:150–60.CrossRefGoogle Scholar
-
McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30:227–39.CrossRefGoogle Scholar
-
Merwick Á, Minhas Z, Curtis C, Thom M, Choi D, Mummery C. Intradural extramedullary spinal candida infection. Pract Neurol. 2015;15:400–4.CrossRefGoogle Scholar
-
Mukhopadhyay S, Katzenstein ALA. Biopsy findings in acute pulmonary histoplasmosis: unusual histologic features in 4 cases mimicking lymphomatoid granulomatosis. Am J Surg Pathol. 2010;34:541–6.CrossRefGoogle Scholar
-
Naggie S, Perfect JR. Molds: hyalohyphomycosis, phaeohyphomycosis, and zygomycosis. Clin Chest Med. 2009;30:337–53.CrossRefGoogle Scholar
-
Neilson JB, Fromtling RA, Bulmer GS. Cryptococcus neoformans: size range of infectious particles from aerosolized soil. Infect Immun. 1997;17:634–8.Google Scholar
-
Nemecek JC, Wuthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–8.CrossRefGoogle Scholar
-
Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc. 2008;83:343–9.CrossRefGoogle Scholar
-
Patel A, Gattuso JP, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256–61.CrossRefGoogle Scholar
-
Perdigao J, Rojas R, Verzelli LF, Castillo M. Fungal infections of the central nervous system. Semin Roentgenol. 2004;39(4):505–18.CrossRefGoogle Scholar
-
Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A. Contribution of the (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2008;46:1009–13.CrossRefGoogle Scholar
-
Posch W, Heimdörfer D, Wilflingseder D, Lass-Flörl C. Invasive candidiasis: future directions in non-culture based diagnosis. Expert Rev Anti Infect Ther. 2017;15(9):829–38. https://doi.org/10.1080/14787210.2017.1370373.CrossRefPubMedGoogle Scholar
-
Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10(Suppl 1):31–47. https://doi.org/10.1111/j.1470-9465.2004.00843.x.CrossRefPubMedGoogle Scholar
-
Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–81.CrossRefGoogle Scholar
-
Raman-Sharma R. Fungal infections of the nervous system: current perspective and controversies in management. Int J Surg. 2010;8(8):591–601.CrossRefGoogle Scholar
-
Read RW, Zhang J, Albini T, Evans M, Rao NA. Splendore-Hoeppli phenomenon in the conjunctiva: immunohistochemical analysis. Am J Ophthalmol. 2005;140:262–6.CrossRefGoogle Scholar
-
Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301.CrossRefGoogle Scholar
-
Riscili BP, Wood KL. Noninvasive pulmonary Aspergillus infections. Clin Chest Med. 2009;30:315–35.CrossRefGoogle Scholar
-
Rixford E, Gilchrist TC. Two cases of protozoon (coccidioidal) infection of the skin and other organs. Johns Hopkins Hosp Rep. 1896;1:209–68.Google Scholar
-
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53.CrossRefGoogle Scholar
-
Roilides E, Antachopoulos C, Simitsopoulou M. Pathogenesis and host defence against Mucorales: the role of cytokines and interaction with antifungal drugs. Mycoses. 2014;57(Suppl 3):40–7. https://doi.org/10.1111/myc.12236.CrossRefPubMedGoogle Scholar
-
Salvatore CM, Chen TK, Toussi SS, De La Mora P, Petraitiene R, Finkelman MA, Walsh TJ. (1→3)-β-D-Glucan in cerebrospinal fluid as a biomarker for Candida and Aspergillus infections of the central nervous system in pediatric patients. J Pediatr Infect Dis Soc. 2016;5(3):277–86. https://doi.org/10.1093/jpids/piv014.CrossRefGoogle Scholar
-
Sargent J, O’Marcaigh A, Smith O, Butler K, Gavin P, O’Sullivan M. Candida albicans-associated necrotizing vasculitis producing life-threatening gastrointestinal hemorrhage. Hum Pathol. 2010;41:602–4.CrossRefGoogle Scholar
-
Sarosi GA, Davies SF. Blastomycosis. State of the art. Am Rev Respir Dis. 1979;120:911–38.PubMedGoogle Scholar
-
Saubolle MA. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:301–14.CrossRefGoogle Scholar
-
Schuetz AN, Walsh TJ. Importance of fungal histopathology in immunocompromised pediatric patients: it’s not just “Aspergillus” anymore. Am J Clin Pathol. 2015;144(2):185–7. https://doi.org/10.1309/AJCPE3NSJ2RYLENS.CrossRefPubMedGoogle Scholar
-
Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol. 2018;17(4):362–72. https://doi.org/10.1016/S1474-4422(18)30030-9.CrossRefPubMedPubMedCentralGoogle Scholar
-
Segal E, Elad D. Candidiasis. In: Mahy BW, Meulen Borriello VP, Murray PR, Funke G, Kaufmann SH, Steward HM, Merz WG, Hay RJ, Cox F, Wakelin D, Gillespie SH, Despommier DD, editors. Topley & Wilson’s microbiology and microbial infections. New York: Wiley; 2010. https://doi.org/10.1002/9780470688618.taw0157.CrossRefGoogle Scholar
-
Sherif R, Segal BH. Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr Opin Pulm Med. 2010;16:242–50.PubMedPubMedCentralGoogle Scholar
-
Shukla S, Singh S, Jain M, Kumar Singh S, Chander R, Kawatra N. Paediatric cutaneous blastomycosis: a rare case diagnosed on FNAC. Diagn Cytopathol. 2009;37:119–21.CrossRefGoogle Scholar
-
Sing Y, Ramdial PK. Cryptococcal inflammatory pseudotumors. Am J Surg Pathol. 2007;31:1521–7.CrossRefGoogle Scholar
-
Southern P, Horbul J, Maher D, Davis DA. C. albicans colonization of human mucosal surfaces. PLoS One. 2008;3:1–9.CrossRefGoogle Scholar
-
Sundaram C, Umabala P, Laxmi V, Purohit AK, Prasad VSSV, Panigrahi M, Sahu BP, Sarathi MV, Kaul S, Borghain R, Meena AK, Jayalakshmi SS, Suvarna A, Mohandas S, Murthy JMK. Pathology of fungal infections of the central nervous system: 17 years’ experience from Southern India. Histopathology. 2006;49:396–405. https://doi.org/10.1111/j.1365-2559.2006.02515.x.CrossRefPubMedPubMedCentralGoogle Scholar
-
Swartzentruber S, Rhodes L, Kurkjian K, Zahn M, Brandt ME, Connolly P, Wheat LJ. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis. 2009;49:1878–82.CrossRefGoogle Scholar
-
The Library of Victoria University, Toronto. Epidemics, hippocrates. Available at: https://archive.org/stream/L477HippocratesVII.EpidemicsLoebClassicalLibrary/L477-Hippocrates%20VII.%20Epidemics%20%28Loeb%20Classical%20Library%29_djvu.txt.
-
Vazquez J, Sobel J. Candidiasis. In: Kaufman C, Pappas P, Sobel J, Dismukes W, editors. Essentials of clinical mycology. 2nd ed. Oxford: Oxford University Press; 2011. p. 167–206.CrossRefGoogle Scholar
-
Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016;30:207–27. https://doi.org/10.1016/j.idc.2015.10.009.CrossRefPubMedPubMedCentralGoogle Scholar
-
Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017;13:13–24.CrossRefGoogle Scholar
-
Zimmermann J, Guresir A, Nelles M, Guresir E. Rapid development and rupture of a cerebral mycotic aneurysm in Candida infective endocarditis. Intensive Care Med. 2016;42:275–6.CrossRefGoogle Scholar
Copyright information
Mucormycosis
Jump to navigationJump to search
| Mucormycosis | |
|---|---|
| Other names | Zygomycosis[1] |
 |
|
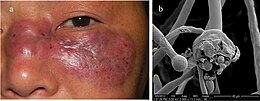
| This patient presented with a case of a periorbital fungal infection known as mucormycosis, or phycomycosis. | |
| Specialty | Infectious disease |
| Causes | Weakened immune system |
| Risk factors | HIV/AIDS, diabetes mellitus, diabetic ketoacidosis, lymphoma, organ transplant, long-term steroid use |
| Treatment | amphotericin B, surgical debridement |
| Prognosis | Poor |
Mucormycosis is any fungal infection caused by fungi in the order Mucorales.[2]:328 Generally, species in the Mucor, Rhizopus, Absidia, and Cunninghamella genera are most often implicated.[3][4]
The disease is often characterized by hyphae growing in and around blood vessels and can be potentially life-threatening in diabetic or severely immunocompromised individuals.
“Mucormycosis” and “zygomycosis” are sometimes used interchangeably.[5] However, zygomycota has been identified as polyphyletic, and is not included in modern fungal classification systems. Also, while zygomycosis includes Entomophthorales, mucormycosis excludes this group.
The condition is informally referred to as black fungus.[6]
Signs and symptoms[edit]
Mucormycosis frequently infects the sinuses, brain, or lungs. While infection of the oral cavity or brain are the most common forms of mucormycosis, the fungus can also infect other areas of the body such as the gastrointestinal tract, skin, and other organ systems.[8] In rare cases, the maxilla may be affected by mucormycosis.[9] The rich blood vessel supply of maxillofacial areas usually prevents fungal infections, although more virulent fungi, such as those responsible for mucormycosis, can often overcome this difficulty.[9]
There are several key signs which point towards mucormycosis. One such sign is fungal invasion into the blood vessels which results in the formation of blood clots and surrounding tissue death due to a loss of blood supply.[10] If the disease involves the brain, then symptoms may include a one-sided headache behind the eyes, facial pain, fevers, nasal congestion that progresses to black discharge, and acute sinusitis along with eye swelling.[11] Affected skin may appear relatively normal during the earliest stages of infection. This skin quickly becomes reddened and may be swollen before eventually turning black due to tissue death.[10] Other forms of mucormycosis may involve the lungs, skin, or be widespread throughout the body; symptoms may also include difficulty breathing, and persistent cough. In cases of tissue death, there may be nausea and vomiting, coughing up blood, and abdominal pain.[8][11]
Risk factors[edit]
Predisposing factors for mucormycosis include HIV/AIDS, uncontrolled diabetes mellitus, cancers such as lymphomas, kidney failure, organ transplant, long term corticosteroid and immunosuppressive therapy, cirrhosis energy malnutrition,[8][9] and deferoxamine therapy.[medical citation needed] Despite this, however, there have been cases of mucormycosis reported with no apparent predisposing factors present.[12]
Corticosteroids are commonly used in the treatment of Covid-19 infection and reduce damage caused by the body’s own immune system during a coronavirus infection. They are immunosuppressant and increase blood sugar levels in both diabetics and non-diabetic patients. It is thought that both these effects may contribute to cases of mucormycosis.[13][14][15][16]
Diagnosis[edit]
As swabs of tissue or discharge are generally unreliable, the diagnosis of mucormycosis tends to be established with a biopsy specimen of the involved tissue.[citation needed]
Treatment[edit]
If mucormycosis is suspected, amphotericin B therapy should be immediately administered due to the rapid spread and high mortality rate of the disease. Amphotericin B is usually administered for an additional 4–6 weeks after initial therapy begins to ensure eradication of the infection. Isavuconazole was recently FDA approved to treat invasive aspergillosis and invasive mucormycosis.[17]
After administration of either amphotericin B or posaconazole, surgical removal of the “fungus ball” is indicated. The disease must be monitored carefully for any signs of reemergence.[8][18]
Surgical therapy can be very drastic, and in some cases of disease involving the nasal cavity and the brain, removal of infected brain tissue may be required. In some cases surgery may be disfiguring because it may involve removal of the palate, nasal cavity, or eye structures.[11] Surgery may be extended to more than one operation.[8] It has been hypothesized that hyperbaric oxygen may be beneficial as an adjunctive therapy because higher oxygen pressure increases the ability of neutrophils to kill the organism.[10]
Prognosis[edit]
In most cases, the prognosis of mucormycosis is poor and the disease has varied mortality rates depending on its form and severity. In the rhinocerebral form, the mortality rate is between 30% and 70%, whereas disseminated mucormycosis presents with the highest mortality rate in an otherwise healthy patient, with a mortality rate of up to 90%.[10] Patients with AIDS have a mortality rate of almost 100%.[18] Possible complications of mucormycosis include the partial loss of neurological function, blindness and clotting of brain or lung vessels.[11]
Epidemiology[edit]
Mucormycosis is a very rare infection, and as such, it is hard to note histories of patients and incidence of the infection.[8] However, one American oncology center revealed that mucormycosis was found in 0.7% of autopsies and roughly 20 patients per 100,000 admissions to that center.[18] In the United States, mucormycosis was most commonly found in rhinocerebral form, almost always with hyperglycemia and metabolic acidosis (e.g. DKA).[12] In most cases the patient is immunocompromised, although rare cases have occurred in which the subject was not; these are usually due to a traumatic inoculation of fungal spores. Internationally, mucormycosis was found in 1% of patients with acute leukemia in an Italian review.[8]
Outbreaks[edit]
Not every hospital in the USA is required to publicize details of infectious outbreaks which occur within their facilities. In 2014, details of a lethal mucormycosis outbreak[19] which occurred in 2008 emerged after television and newspaper reports responded to an article in a pediatric medical journal.[20] Contaminated hospital linen was found to be spreading the infection. A 2018 study found many freshly laundered hospital linens delivered to U.S. transplant hospitals were contaminated with Mucorales.[21]
A cluster of infections occurred in the wake of the 2011 Joplin tornado. As of July 19, a total of 18 suspected cases of cutaneous mucormycosis had been identified, of which 13 were confirmed. A confirmed case was defined as 1) necrotizing soft-tissue infection requiring antifungal treatment or surgical debridement in a person injured in the tornado, 2) with illness onset on or after May 22 and 3) positive fungal culture or histopathology and genetic sequencing consistent with a Mucormycete. No additional cases related to that outbreak have been reported since June 17. Ten patients required admission to an intensive-care unit, and five died.[22][23]
Cutaneous mucormycosis has been reported after previous natural disasters; however, this is the first known cluster occurring after a tornado. None of the infections were found in persons cleaning up debris; instead it is believed transmission occurred through penetrating injuries inflicted by contaminated objects (e.g. splinters from a woodpile).[24]
During the COVID-19 pandemic, a number of cases linked to immunosuppressive treatment for COVID-19 were reported in India. In Ahmedabad, 44 cases including nine deaths were reported by mid-December 2020. Cases were also reported in Mumbai and Delhi.[25] In 2021, more cases were also reported throughout India.[6]
See also[edit]
References[edit]
- ^ RESERVED, INSERM US14– ALL RIGHTS. “Orphanet: Zygomycosis”. www.orpha.net. Retrieved June 28, 2019.
- ^ James, William D.; Berger, Timothy G.; et al. (2006). Andrews’ Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ^ Rinaldi M.G. (1989). “Zygomycosis”. Infect Dis Clin North Am. 3 (1): 19–41. doi:10.1016/S0891-5520(20)30244-0. PMID 2647832.
- ^ Lee F.Y.; Mossad S.B.; Adal K.A. (1999). “Pulmonary mucormycosis: the last 30 years”. Arch Intern Med. 159 (12): 1301–9. doi:10.1001/archinte.159.12.1301. PMID 10386506.
- ^ Staff Springfield News-Leader (June 10, 2011) “Aggressive fungus strikes Joplin tornado victims” Seattle PI, Hearst Communications Inc.
- ^ Jump up to:a b “Mucormycosis: The ‘black fungus’ maiming Covid patients in India”. May 9, 2021 – via www.bbc.com.
- ^ Ran Yuping (2016). “Observation of Fungi, Bacteria, and Parasites in Clinical Skin Samples Using Scanning Electron Microscopy”. In Janecek, Milos; Kral, Robert (eds.). Modern Electron Microscopy in Physical and Life Sciences. InTech. doi:10.5772/61850. ISBN 978-953-51-2252-4.
- ^ Jump up to:a b c d e f g Nancy F Crum-Cianflone; MD MPH. “Mucormycosis”. eMedicine. Retrieved May 19, 2008.
- ^ Jump up to:a b c Auluck A (2007). “Maxillary necrosis by mucormycosis. a case report and literature review” (PDF). Med Oral Patol Oral Cir Bucal. 12 (5): E360–4. PMID 17767099. Retrieved May 19, 2008.
- ^ Jump up to:a b c d Spellberg B, Edwards J, Ibrahim A (2005). “Novel perspectives on mucormycosis: pathophysiology, presentation, and management”. Clin. Microbiol. Rev. 18 (3): 556–69. doi:10.1128/CMR.18.3.556-569.2005. PMC 1195964. PMID 16020690.
- ^ Jump up to:a b c d “MedlinePlus Medical Encyclopedia: Mucormycosis”. Retrieved May 19, 2008.
- ^ Jump up to:a b Roden MM, Zaoutis TE, Buchanan WL, et al. (September 2005). “Epidemiology and outcome of Mucormycosis: a review of 929 reported cases”. Clin. Infect. Dis. 41 (5): 634–53. doi:10.1086/432579. PMID 16080086.
- ^ Biswas, Soutik (May 9, 2021). “Mucormycosis: The ‘black fungus’ maiming Covid patients in India”. BBC News. British Broadcasting Corporation. Retrieved May 11, 2021.
- ^ Koehler, Philipp; Bassetti, Matteo; Chakrabarti, Arunaloke; Chen, Sharon C A; Colombo, Arnaldo Lopes; Hoenigl, Martin; Klimko, Nikolay; Lass-Flörl, Cornelia; Oladele, Rita O; Vinh, Donald C; Zhu, Li-Ping (December 2020). “Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance”. The Lancet Infectious Diseases. doi:10.1016/s1473-3099(20)30847-1. ISSN 1473-3099.
- ^ “Mucormycosis: The ‘black fungus’ maiming Covid patients in India”. BBC News. May 9, 2021. Retrieved May 11, 2021.
- ^ Garg, Deepak; Muthu, Valliappan; Sehgal, Inderpaul Singh; Ramachandran, Raja; Kaur, Harsimran; Bhalla, Ashish; Puri, Goverdhan D.; Chakrabarti, Arunaloke; Agarwal, Ritesh (May 1, 2021). “Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature”. Mycopathologia. 186 (2): 289–298. doi:10.1007/s11046-021-00528-2. ISSN 1573-0832. PMC 7862973. PMID 33544266.
- ^ Lyndsay Mayer. “Mucormycosis”. Food and Drug Administration. Retrieved April 5, 2017.
- ^ Jump up to:a b c Rebecca J. Frey. “Mucormycosis”. Health A to Z. Archived from the original on May 18, 2008. Retrieved May 19, 2008.
- ^ Catalanello, Rebecca (April 16, 2014). “Mother believes her newborn was the first to die from fungus at Children’s Hospital in 2008”. NOLA.com.
- ^ “5 Children’s Hospital patients died in 2008, 2009 after contact with deadly fungus”.
We acknowledge that Children’s Hospital is Hospital A in an upcoming article in The Pediatric Infectious Disease Journal. The safety and well-being of our patients are our top priorities, so as soon as a problem was suspected, the State Health Department and CDC were notified and invited to assist in the investigation. The hospital was extremely aggressive in trying to isolate and then eliminate the source of the fungus.
- ^ Sundermann, Alexander; et al. (2018). “How Clean Is the Linen at My Hospital? The Mucorales on Unclean Linen Discovery Study of Large United States Transplant and Cancer Centers”. Clinical Infectious Diseases. 68 (5): 850–853. doi:10.1093/cid/ciy669. PMC 6765054. PMID 30299481.
- ^ Williams, Timothy (June 10, 2011) Rare Infection Strikes Victims of a Tornado in Missouri. New York Times.
- ^ Neblett Fanfair, Robyn; Benedict, Kaitlin; Bos, John; Bennett, Sarah D.; Lo, Yi-Chun; Adebanjo, Tolu; Etienne, Kizee; Deak, Eszter; Derado, Gordana; Shieh, Wun-Ju; Drew, Clifton; Zaki, Sherif; Sugerman, David; Gade, Lalitha; Thompson, Elizabeth H.; Sutton, Deanna A.; Engelthaler, David M.; Schupp, James M.; Brandt, Mary E.; Harris, Julie R.; Lockhart, Shawn R.; Turabelidze, George; Park, Benjamin J. (2012). “Necrotizing Cutaneous Mucormycosis after a Tornado in Joplin, Missouri, in 2011”. New England Journal of Medicine. 367(23): 2214–25. doi:10.1056/NEJMoa1204781. PMID 23215557.
- ^ Fanfair, Robyn Neblett; et al. (July 29, 2011). “Notes from the Field: Fatal Fungal Soft-Tissue Infections After a Tornado – Joplin, Missouri, 2011”. MMWR Weekly. 60 (29): 992.
- ^ “‘Black’ Fungal Disease that Causes Blindness, Death Strikes Guj after Covid-19; Kills 9 in Ahmedabad”. News18. December 18, 2020. Retrieved December 18, 2020.
External links[edit]
| Classification | |
|---|---|
| External resources |
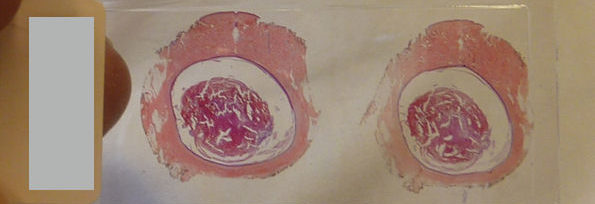

 Views Today : 6366
Views Today : 6366