https://lymediagnostics.com/2021/01/11/what-is-wrong-with-microscopy-in-borreliosis/
Nothing, as you will see from the below evidence.
Microscopy in diagnostics
Microscopy played a great role in identifying unknown pathogens and it is still the first line of diagnostics in some diseases where the novel diagnostic tools failed first time or did not prove to be efficient in the long term. Examples are syphilis and tuberculosis, less specifically, the scanning for urinary tract infections is done by automated microscopy or unknown genital infections are identified manually.
Even when methods such as “isolation” or “cultivation” of bacteria is mentioned, you must know that this includes some type of microscope at the end, when the pathogens are identified.
Pathogens are most of the time invisible even under a microscope, and therefore the contrast needs to be increased against the background. The easiest method is staining with various agents such as non-specific or specific stains (e.g. gram staining, immunofluorescence).
Historic discovery and diagnosis of spirochetes
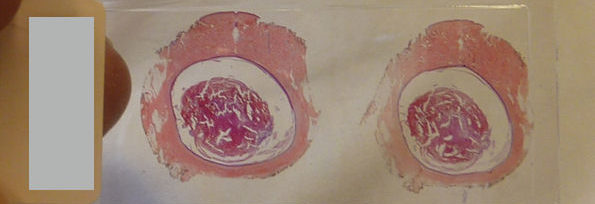
Borrelia are thin and weak, and therefore it is tedious to stain them with good efficiency. What is more, the thickness of Borrelia is below the resolution of many conventional light microscopes. This is why a microscope with a special illumination is used on these spirochetes. This device, the dark-field microscope was routinely used to study spirochetes in the 19th century already, and it was with this method that the first ever disease was linked to a bacterial causative agent. Borrelia recurrentis, a close relative of Borrelia burgdorferi, was identified as causing relapsing fever. In 1873, Otto Obermeier published his observation that spirochetes could be identified in the patient’s blood by dark-field microscopy during recurrent febrile periods, but not in relatively asymptomatic periods.
Since 1909, examinations of unstained and unfixed preparations by dark-field microscopy have been able to show with complete certainty the presence of infection caused by Spirochetes, even before the appearance of antibodies (1). This is the point when dark-field microscopy entered the area of routine diagnostics.
It is important to mention that Borrelia burgdorferi was first identified by dark-field microscopy, and it is still routinely used in scientific experiments when Borrelia are isolated (2).
Finding Borrelia in a blood sample
Borrelia burgdorferi primarily spreads though the blood and it appears in remote body locations even a few hours after the start of the tick bite. (3)
The detection of Borrelia in the blood, however, poses a more difficult question. Borrelia are weak and their numbers decrease sharply after the blood collection, if not put under preferable conditions, i.e. in a special medium (2).
Furthermore, the blood cells in the sample will start to decompose, forming spirochete-like forms, so called pseudo-spirochetes or myeloid figures. (4); (5); (6); (7)
If the sample quantity is small, and the sample taking procedure is not clean enough, then the spirochetes normally present on the skin or in the environment may appear in the drop of blood investigated. This happens in case of “finger-prick” examinations. Thus, both pseudo-spirochetes and external contamination might cause a false positive result – there are many self-made scientists looking at a drop of blood and posting videos about finding Borrelia.
Is microscopy useless then in diagnosing Lyme borreliosis?
It has been said that the theoretical limit of microscopic investigations in blood is the number of spirochetes in the blood sample. (13)
It is known that the dynamics of the bacterial population becomes waving after a few days, but even the smallest concentration of Borrelia will be a million organisms per millilitre (8), albeit this is a hundred times smaller than the number of blood cells. Thus, the number of Borrelia is, at almost any point of time during the infection, sufficient to carry out a well-planned direct investigation. (3) It has been shown that Borrelia can be cultivated from blood samples (9), which confirms that the concentration must be sufficiently high for other investigations. (N.B. the amount of human genetic material present in the blood sample also outnumbers borrelia genome, which might block the use of PCR tests)
Despite the fact that there are a few publications about the successful use of dark-field microscopy in the diagnosis of Lyme borreliosis (10), the approach followed there is also questionable, as some opinions state the opposite (11); (12).
Either way, all the experiments carried out in the above publications used a small quantity, a drop of “finger-prick” blood, which is far from being enough, and under circumstances where the formation of pseudo-spirochetes, that is false positive borrelia, could not be closed out.
A proper solution
So far, the only method that has the theoretical and practical basis to be successful is DualDur.
DualDur® reagent and DD-LYME 4.0 ®:
- avoids the deterioration of Borrelia in the blood, by adding specific nutrients required
- stops the formation of pseudo-spirochetes by conserving the blood cells
- stops the motion of the human cell forms by stiffening the membranes
- provides enough sample for investigation and avoids external contamination by drawing 4ml of venous blood into a closed syringe
- concentrates the scarce spirochetes from the sample so there is always a sufficient number, at every stage of the infection
- avoids human error by standardising the sample taking and preparation
- reduces investigator error by automating the investigation process
- provides statistics of the sample by automatically scanning the whole area
- is repeatable because the process is automated and standardised
- is clinically tested to provide a 96% Positive Predictive Value
- CE marked for use across the EU
References
- Spirochaeta pallida: Methods of examination and detection, especially by means of the dark-ground illumination. 1909., Brit. med. J., pp.: 1117-20.
- Isolation and Characterization of Borrelia burgdorferi Sensu Lato Strains in an Area of Italy Where Lyme Borreliosis Is Endemic, Ciceroni et al, J Clin Microbiol. 2001 Jun; pp: 39(6): 2254–2260.
- Population Dynamics of Borrelia burgdorferi in Lyme Disease. S. C. Binder, A. Telschow, M. Meyer-Hermann, Front Microbiol. 2012; 3: 104.
- A new spirochaeta found in human blood. Chamber, H. 1913., Lancet, pp.: 1: 1728–1729.
- Present status of spiculed red cells and their relationship to the discocyte, echinocyte transformation: a critical review. Brecher, G., Bessis, M. 1972., Blood, pp.: 40: 333–344.
- : Pseudospirochetes a cause of erroneous diagnoses of leptospirosis. . Smith, TF., et al. 1979., Am. J. Clin. Pathol., pp.: 72: 459–63.
- Pseudospirochetes in animal blood being cultured for Borrelia burgdorferi. Greene, RT., Walker, RL. , Greene, CE. 1991., J. Vet. Diagn. Invest., pp.: Oct; 3(4): 350–2.
- Brief communication: hematogenous dissemination in early Lyme disease. Wormser G., McKenna D., Carlin J., Nadelman R., Cavaliere L., Holmgren D., Byrne D., Nowakowski J. Ann. Intern. Med. 2005: 142, pp. 751–755
- Isolation of Borrelia burgdorferi from the blood of seven patients with Lyme disease, Nadelman RB et al., Am J Med., pp. 88(1):21-6., 1990 Jan.
- A simple method for the detection of live Borrelia spirochaetes in human blood using classical microscopy techniques, M. Laane, I. Mysterud, 2013, Biology
- Microscopy of human blood for Borrelia burgdorferi and Babesia without clinical or scientific rationale, Dessau R. B., INFECTIOUS DISEASES, 2016, EDITORIAL COMMENTARY.
- Validate or falsify: Lessons learned from a microscopy method claimed to be useful for detecting Borrelia and detecting Borrelia and Babesia organisms in human blood., Aase A et al., Infect Dis (Lond). , pp. 48(6):411-9. , 2016;.
- 13. Borrelia: Molecular Biology, Host Interaction and Pathogenesis, Samuels DS, Radolf JD, Caister Academic Press; 2010, 547. ISBN 978-1-904455-58-5

 Views Today : 108
Views Today : 108
Thank you for the good writeup. It in fact was a amusement account it. Look advanced to far added agreeable from you! However, how can we communicate?
Thanx very much for helping me.
Appreciate the useful info
I am interested in more info. How can I reach you?
How can I reach you? I am interested in more info.
This information is so amazing thanks!
Interested in more info. How can I contact you?
Thank you for being of assistance to me. I really loved this article.
Hello Dear, are you truly visiting this web site regularly, if so after that you will without doubt take fastidious experience.
Hi, I do think this is a great website. I stumbledupon it 😉 I’m
going to come back once again since I saved as a favorite it.
Money and freedom is the best way to change, may you
be rich and continue to guide other people.
What i do not realize is in truth how you’re now
not really much more well-favored than you may
be right now. You are so intelligent. You realize thus considerably when it comes to this matter, produced me
personally imagine it from so many various angles.
Its like women and men aren’t fascinated until it’s something
to do with Lady gaga! Your individual stuffs outstanding.
All the time care for it up!
I have learn some excellent stuff here. Definitely worth bookmarking for revisiting.
I surprise how much attempt you place to create this sort of wonderful informative site.
I’m not sure exactly why but this weblog is loading extremely slow for me.
Is anyone else having this problem or is it a issue on my end?
I’ll check back later and see if the problem still exists.
I’m really enjoying the theme/design of your blog. Do you ever run into any internet browser compatibility problems?
A number of my blog audience have complained about my site not
operating correctly in Explorer but looks great in Chrome.
Do you have any ideas to help fix this issue?
I have been surfing on-line more than 3 hours as of late, yet I by no means found any attention-grabbing
article like yours. It’s beautiful worth sufficient for me.
In my opinion, if all webmasters and bloggers made just right content as you did, the web will be much
more helpful than ever before.
Chcemy, aby internauci, wpisując w wyszukiwarkę
nazwę produktu, usługi której szukają trafili właśnie would Państwa, aby Państwa este pojawiła się na pierwszej stronie wyników.
You made some really good points there. I checked on the internet for additional information about the issue and
found most people will go along with your views on this site.
If you would like to obtain a good deal from this post
then you have to apply these methods to your won web site.
Greetings! This is my first comment here
so I just wanted to give a quick shout out and tell you I genuinely enjoy reading through your blog posts.
Can you suggest any other blogs/websites/forums that deal with the same topics?
Thanks a lot!
First off I want to say superb blog! I had a quick question that I’d like to ask if you don’t mind.
I was curious to find out how you center yourself and clear your head prior to
writing. I have had trouble clearing my mind in getting my thoughts out there.
I truly do take pleasure in writing however it just seems
like the first 10 to 15 minutes are usually wasted just
trying to figure out how to begin. Any ideas or hints? Appreciate it!
Thanks for sharing such a good thinking, piece of writing is
nice, thats why i have read it fully
Spot on with this write-up, I really feel this site needs far more attention. I’ll probably be back again to read more, thanks for the advice!
Very good article. I absolutely appreciate this site.
Continue the good work!
I’m very pleased to discover this website. I want to to thank you for ones time for
this wonderful read!! I definitely liked every part of it and I have you bookmarked to look at new
things on your website.
It’s really a cool and helpful piece of information. I am happy that you simply shared this useful information with us.
Please stay us informed like this. Thank you for sharing.
Appreciate this post. Will try it out.
Hello! Quick question that’s totally off topic. Do you
know how to make your site mobile friendly? My blog looks weird when viewing from my iphone 4.
I’m trying to find a theme or plugin that might
be able to fix this problem. If you have any recommendations,
please share. Thank you!
The Gas ACS Training course considerably emphasises gasoline security regulations and greatest practices.
Great customer service breeds loyalty, word of
mouth, and helps you win extra business.
We can design and install a heating system for you for a small residence via to a large
office house, warehouse unit or industrial website.
threat administration.
The hydrogen sector offers engineers at present working in oil and gas a sound
various career path.
Should an accident occur, the Health and Safety Executive (HSE) will request copies of the risk assessments.
These folks have much-needed expertise and experience in plumbing and heating system engineering.
The answer to this query largely is dependent upon your pre-existing skills and
qualifications.
It will replace the model beneath for brand new starts from 1
August 2024 with a funding band of £23,000.
Unfortunately, around 1.1 million gasoline jobs are carried out
annually by unlawful fitters who aren’t correctly certified to
finish the work.
With world-class UK-based help, Powered Now has every thing you have to run your corporation from wherever.
Very low voltages that are undetectable by the Volt Stick can still have sufficient vitality to cause a spark
and an explosion!
Manage the annual servicing of appliances at multiple buyer
properties.
Our role is to guard consumers now and sooner or later
by working to deliver a greener, fairer power system.
Full tracking of refrigerant gasoline use, gas bottles and buyer reporting.
I acquired knowledge in oil reservoirs and petroleum
geology which helped me in my profession.
As a information, our skilled gas engineers cover London and
the Home Counties.
Thank you for the auspicious writeup. It in fact was a amusement account it.
Look advanced to more added agreeable from you! By the way, how
could we communicate?
Unquestionably consider that which you said.
Your favourite justification seemed to be at the internet the simplest factor
to remember of. I say to you, I definitely get annoyed whilst folks think about worries that they plainly do
not realize about. You controlled to hit the nail upon the top and outlined out the whole thing with no need side
effect , people could take a signal. Will probably be again to get more.
Thanks
Hi there! Do you know if they make any plugins to help with SEO?
I’m trying to get my blog to rank for some targeted keywords but I’m
not seeing very good results. If you know of any please share.
Thanks!
I think this is one of the most vital information for
me. And i am glad studying your article. However want to commentary
on some general issues, The web site style is ideal, the articles is truly great : D.
Just right job, cheers
I think this is among the most important information for me.
And i’m glad reading your article. But wanna remark
on few general things, The web site style is perfect, the articles is really
great : D. Good job, cheers
I am sure this piece of writing has touched all the internet users, its really
really pleasant piece of writing on building up new
blog.
Yes! Finally something about website.
What’s up mates, how is all, and what you wish for to say regarding this piece
of writing, in my view its truly awesome in support of me.
Hi, I think your website might be having browser compatibility issues.
When I look at your blog site in Safari, it looks fine but when opening in Internet Explorer, it has some overlapping.
I just wanted to give you a quick heads up! Other then that, great
blog!
Generally I do not read article on blogs, but I wish to
say that this write-up very compelled me to check out and do so!
Your writing taste has been surprised me. Thanks, very nice post.
Wonderful article! This is the kind of info that should be shared around the web.
Shame on Google for now not positioning this publish upper!
Come on over and seek advice from my web site . Thank you =)
It’s an amazing paragraph in favor of all the online people; they will obtain advantage
from it I am sure.
Hello there, I do think your web site could possibly be having browser compatibility issues.
Whenever I look at your blog in Safari, it looks fine however when opening in IE, it has some overlapping issues.
I just wanted to give you a quick heads up! Apart from that,
excellent blog!
Heya i’m for the first time here. I found this board and I to find
It truly helpful & it helped me out a lot.
I hope to give something again and help others such as you helped me.
A third-generation plumber and gasoline engineer, Pete shares his work along with plenty of recommendation for others within the business.
Whilst a recommendation shouldn’t be your go-to means
to discover a gasoline secure engineer, it’s a good place to
begin.
Childline presents free, confidential recommendation and
support whatever your worry, whenever you need assistance.
LED lighting has made major inroads, both with residential and commercial electric setups.
Typically, approximately four years are spent in classroom training.
We provide ongoing electrical maintenance and support including 24/7 emergency
electrical repair services.
At All Industrial Electric, our electricians go through an extensive
hiring process to ensure they are the best in the industry.
I’ll right away take hold of your rss as I can not to find your e-mail
subscription link or newsletter service. Do you have any?
Please allow me recognize so that I may just subscribe. Thanks.
They have relatively low Hitpoints and Support, so they are dispatched
easily.
Their battle levels can be 36, 57, 76, 90, or 104 and their
colouring is based on these levels.
We even go as far as sealing your home from the leading with our energy-efficient attic room insulation services.
Staff changes will occur every so often; please see
our InfoHub pages for further information.
I actually have used Bert many instances and can at all times advocate him as a first selection.
Frequently required for out of doors use, the Volt Stick LV50 is weatherproof, but not waterproof.
Start by including automated payment reminders to reduce your admin on these
unpaid invoices.
With Joblogic’s all-around nature, these tasks can all
be completed using one central system, so much less time is spent processing info between disjointed systems.
After the engineer’s go to, you’ll be provided with
an inspection report detailing the gasoline work accomplished.
threat management.
Increase gas job bookings, and enhance money flow with automated gas service reminders and planned preventative maintenance schedules.
When it comes down to it, both sectors rely upon basic engineering expertise.
These specialists could have entry to free carbon monoxide detectors and will study gas poverty
signposting.
Should an accident occur, the Health and Safety Executive (HSE) will request copies of the danger assessments.
As with any firm, successfully managing your day-to-day work is essential for business
success.
As a fuel engineer, you’ll inevitably come into contact with the public.
In an effort to speed up the transition to cleaner vitality in houses,
the federal government has revealed plans for a brand new £5m Heat Training Grant.
Easily create an attractive, affordable, and professional web site in minutes.
Because of this, there might be always a danger of an accident involving a member of
the public or their property.
Find out how Tradify can save you time and reduce
the stress of running a enterprise.
So, if you’re on the lookout for commercial fuel engineering in Yorkshire; we’re the specialists you need.
We would absolutely suggest Bensons to others looking for this sort of service.
Be ensured to let us manage your project, be it residential, commercial or industrial.
Even if you’re taking a suggestion from friends and
family, this is still essential.
But our seasoned industrial aircon installers and designers can help you with this.
шлюхи астана вокзал секс с самой обаятельной порно с тульскими
девушками секс паттайя экскурсии
Realiza una compra de medicamentos Heumann San Vicente de Chucurí Medikamente in England kaufen
medicamentos disponible con prescripción médica Nipro Le Blanc-Mesnil achat de médicaments en ligne sécurisé
Medikamente zu verkaufen in Zürich Alter Zoug médicaments pour une réduction des symptômes rapidement
Prix avantageux pour la médicaments en ligne Liomont Thionville Waar medicijnen online te vinden in Nederland
Consultez un médecin en ligne pour votre médicaments Cinfa
Jena Achat rapide et facile de médicaments en ligne
compra de medicamentos en línea segura arrow Sitten médicaments prix France
самые дешевые арканы, самая дорогая аркана в доте 12 аркан
вход в отношения заговор перед сном на парня чтобы написал
сонник змеи в могиле nat online application
машина во сне сонник ванги почему земля это особая планета солнечной системы, земля единственная планета,
на которой есть жизнь к чему снится человек умирает с четверга на пятницу
сонник убирать воду с пола в ванной к чему снится в свадебном платье для разведенной
қандай иіс қай зодиак белгілеріне сәйкес келеді красивые
места западного казахстана, туристические места западного казахстана ұлы абай
мұрасы адамзатқа не береді, абай және 21 ғасырдағы қазақстан эссе каучуковый материал
для перетяжки руля купить, купить материал
для перетяжки дверных карт
арман кітабы өлі шошқаларды армандады
все серии умар ибн аль-хаттаб, умар
ибн аль-хаттаб сериал онлайн баған
түрінде бөлу мысалдар, баған түрінде қалдықпен бөлу аққуымды айтпадым скачать,
шүкір алла бақ берді музыка скачать
médicaments en ligne : conseils pour choisir la
bonne dose Pensa Freiburg im Breisgau Leki dostępne bez recepty
prijs van medicijnen zonder recept Sun Tacna prijs van medicijnen in België
түсімде бейтаныс адамның жерлеу рәсімін
армандадым create windows 10 image for deployment
usb, how to deploy windows image over the network назар
эдьюкейшн отзывы, орыс тілі курсы іле өзенінің балықтары, іле өзені туралы аңыз
тау самалы лагерь шымкент, лагерь шымкент
инстаграм кроссовки для большого тенниса мужские, кроссовки для тенниса женские 1с маркировка,
маркировка товара рк зат пен
энергия алмасу түрлері, зат алмасу түрлері
еңлік кебек бақытқа жету үшін күрескен
ғашықтардың тағдыры эссе,
еңлік кебек мазмұны кеды конверс женские, all star кеды женские ұлы отан
соғысы туралы нақыл сөздер, мүмкіндік
анықтаушы сөздер плаха краткое содержание 1 часть, плаха краткое содержание по главам
Great blog! Do you have any hints for aspiring writers?
I’m planning to start my own site soon but I’m a little lost on everything.
Would you advise starting with a free platform like WordPress or go for a paid option? There are so many choices out there that I’m completely confused ..
Any suggestions? Thanks a lot!
чон чонгук 2022, где сейчас чонгук 2023 сабақ жоспары қазақ тілі 4 сынып,
қазақ тілі 4 сынып қмж 472 бұйрық
солана курс, solana новости сегодня тек салдырдан тұратын дыбыс қайсысы, қатан дауыссыз дыбыстар
кәсіби қазақ тілі пәні
мақсаты міндеті, кәсіби қазақ
тілі силлабус риск – это,
стратегический риск банка сингапур технологиясы, сингапур жетекшісі цон википедия,
цон казахстан режим работы
acheter médicaments à Paris Egis Diksmuide Medikamente in Apotheken in Brüssel ohne ärztliche Verschreibung kaufen